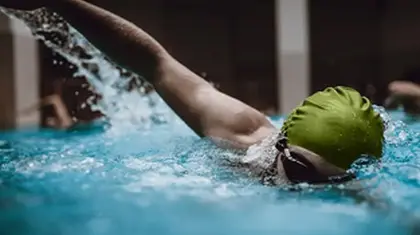Could strawberries help improve cognitive health and mood?
Share on PinterestCan strawberries help improve mood and cognition? Oscar Wong/Getty ImagesStrawberries...
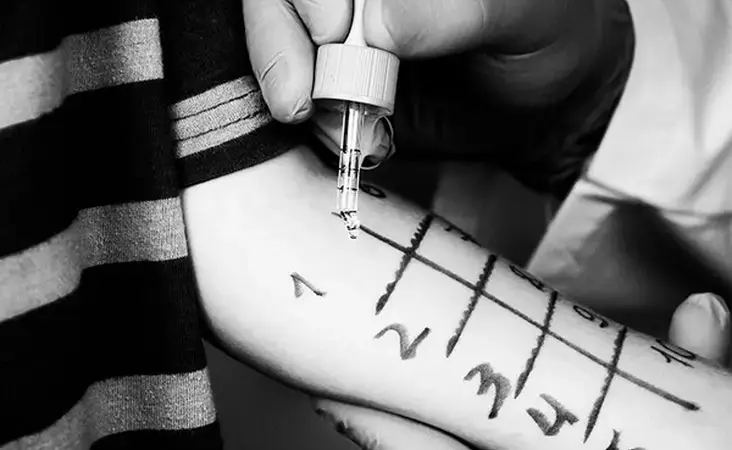
Can semaglutide injections like Ozempic or Wegovy help heart health?
Share on PinterestSemaglutide injections may also have cardiovascular benefits, according to a new trial....
Multiple sclerosis: A low-fat diet may help with fatigue
Share on PinterestIs a low-fat diet helpful in managing MS symptoms? Image credit: Maki Nakamura/Getty...
High cholesterol: Two new drugs show promise in reducing levels
Share on PinterestScientists are looking at two new drugs that target high cholesterol at the genetic...
Cognitive decline: Reduction in hippocampus size can increase risk
Share on PinterestThe pathways in the human brain are similar to a road system. Felix Cesare/Getty ImagesResearchers...
Life expectancy gap: Women in U.S. now outlive men by nearly 6 years
Share on PinterestLife expectancy declined in the United States during the COVID-19 pandemic, especially...
How do alcohol and caffeine affect sleep?
Share on PinterestCaffeine and alcohol may seem like they are aiding sleep, but a new study shows it...
Arthritis risk: How do testosterone levels factor in?
Share on PinterestNew evidence confirms the link between testosterone levels and arthritis risk. Image...
Colorectal risk higher in people with type 2 diabetes
Share on PinterestExperts say the risk of colorectal cancer is higher in people with type 2 diabetes....
High-intensity water exercise can help people with chronic conditions
Share on PinterestSwimming is one high-intensity exercise people can do in the pool. AleksandarGeorgiev/Getty...
New GLP-1 weight loss drug Zepbound approved by FDA: What to know
Share on PinterestEli Lilly’s new weight loss drug, Zepbound, was recently granted FDA approval. Cristina...
Migraine: Discovery of 44 rare genetic variants may lead to treatments
Share on PinterestScientists are looking at 44 gene variants that may be linked to migraine headaches....
LDL cholesterol: Mediterranean diet may not affect levels
Share on PinterestIs following a Mediterranean diet enough to lower cholesterol levels? Image credit:...
Cholesterol: New study may help pave the way for new treatments
Share on PinterestResearchers are studying the ways that cholesterol is absorbed in the body. /Stocksy/Getty...
Type 2 diabetes: How insufficient sleep can increase risk for women
Share on PinterestExperts say a messy bedroom can interfere with healthy sleep habits. Melanie DeFazio/StocksyResearchers...
Parkinson's, Lewy body dementia: PET scan may tell risk
Share on PinterestPET scans may help predict who will go on to develop Parkinson’s disease, dementia...
Breast cancer: How anostrozole can lower risk
Share on PinterestAnastrozole can reduce breast cancer risk after menopause, but does it come with its...
High cholesterol: Genetic testing can help people with family history
Share on PinterestExperts say people with a family history of high cholesterol should get tested. Sergey...
Heart health: How small changes in daily activity can offset sitting
Share on PinterestResearchers say sitting for prolonged periods is detrimental to heart health. DZ FILM/StocksyResearchers...
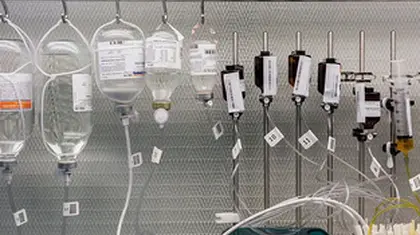
Cardiovascular disease: How food sensitivity may increase the risk
Share on PinterestResearchers say sensitivity to certain foods may increase the risk of heart disease....
How much sleep do you need to prevent cognitive decline?
Share on PinterestThe amount of sleep you need to prevent cognitive decline may depend on whether you’re...
Arrhythmia: Wearable ECG patch may help detect heart conditions
Share on PinterestWearable heart monitor technology may help doctors detect cardiac conditions earlier....
Peanut allergies: Special toothpaste may help reduce reactions
Share on PinterestExperts say a toothpaste with peanut allergens might be a convenient way for people...
Atrial fibrillation: Early onset AFib may increase dementia risk
Share on PinterestExperts say atrial fibrillation should be treated due to the Health conditions it can...
Colon cancer: Could aspirin help slow disease progression?
Share on Pinterestbarleyman/Getty ImagesColorectal cancer, or colon cancer, is the third most common...
Diabetes and mental health: Depression may hasten death
Share on PinterestDepression may hasten death in people with type 2 diabetes. Image credit: Yasser Chalid/Getty...
Epilepsy: How yoga may help reduce seizures, anxiety
Share on PinterestExperts say yoga has many physical and mental health benefits. Maskot/Getty ImagesResearchers...