Drug Detail:Actiq (Fentanyl citrate (oral transmucosal) [ fen-ta-nil-sit-rayt ])
Generic Name: FENTANYL CITRATE 200ug
Dosage Form: lozenge
Drug Class: Opioids (narcotic analgesics)
Important Dosage and Administration Instructions
- Healthcare professionals who prescribe ACTIQ for outpatients must enroll in the TIRF REMS and comply with the requirements of the REMS to ensure safe use of ACTIQ [see Warnings and Precautions (5.7)].
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)].
- It is important to minimize the number of strengths available to patients at any time to prevent confusion and possible overdose.
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with ACTIQ and adjust the dosage accordingly [see Warnings and Precautions (5.1)].
- Instruct patients and caregivers to take steps to store ACTIQ securely and to properly dispose of unused ACTIQ as soon as no longer needed [see Warnings and Precautions (5.1, 5.2), Patient Counseling Information (17)].
- Other TIRF formulations and ACTIQ are not equivalent. DO NOT substitute an ACTIQ prescription for any other TIRF formulation under any circumstances. Do not convert patients on a mcg per mcg basis from any other fentanyl product to ACTIQ [see Warnings and Precautions (5.5)].
2.2 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver and assess the potential need for access to naloxone, both when initiating and renewing treatment with ACTIQ [see Warnings and Precautions (5.1), Patient Counseling Information (17)].
Inform patients and caregivers about the various ways to obtain naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Consider prescribing naloxone, based on the patient’s risk factors for overdose, such as concomitant use of CNS depressants, a history of opioid use disorder, or prior opioid overdose. The presence of risk factors for overdose should not prevent the proper management of pain in any given patient [see Warnings and Precautions (5.1, 5.4, 5.6)].
Consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or overdose.
Initial Dosage
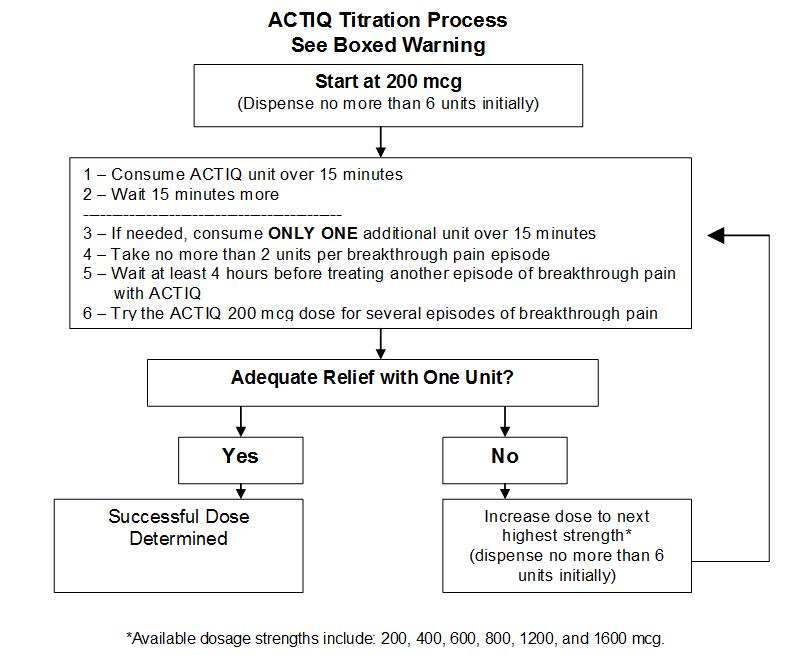
Individually titrate ACTIQ to a dose that provides adequate analgesia and minimizes side effects. The initial dose of ACTIQ to treat episodes of breakthrough cancer pain is always 200 mcg. The ACTIQ unit should be consumed over 15 minutes. Patients should be prescribed an initial titration supply of six 200 mcg ACTIQ units, thus limiting the number of units in the home during titration. Patients should use up all units before increasing to a higher dose to prevent confusion and possible overdose.
Repeat Dosing
- In cases where the breakthrough pain episode is not relieved after 15 minutes after completion of the ACTIQ unit (30 minutes after the start of the unit), patients may take ONLY ONE additional dose using the same strength for that episode. Thus patients should take a maximum of two doses of ACTIQ for any episode of breakthrough pain.
- Patients MUST wait at least 4 hours before treating another episode of breakthrough pain with ACTIQ.
Dose Titration
From an initial dose, closely follow patients and change the dosage strength until the patient reaches a dose that provides adequate analgesia using a single ACTIQ dosage unit per breakthrough cancer pain episode. If signs of excessive opioid effects appear before the unit is consumed, the dosage unit should be removed from the patient’s mouth immediately, disposed of properly, and subsequent doses should be decreased. Patients should record their use of ACTIQ over several episodes of breakthrough cancer pain and review their experience with their physicians to determine if a dosage adjustment is warranted.
In cases where the breakthrough pain episode is not relieved 15 minutes after completion of the ACTIQ unit (30 minutes after the start of the unit), patients may take ONLY ONE additional dose of the same strength for that episode. Thus, patients should take a maximum of two doses of ACTIQ for any breakthrough pain episode.
Patients must wait at least 4 hours before treating another episode of breakthrough pain with ACTIQ. To reduce the risk of overdosing during titration, patients should have only one strength of ACTIQ available at any one time.
Maintenance Dosing
- Once titrated to an effective dose, patients should generally use ONLY ONE ACTIQ unit of the appropriate strength per breakthrough pain episode.
- On those occasions when the breakthrough pain episode is not relieved 15 minutes after completion of the ACTIQ unit, patient may take ONLY ONE additional dose using the same strength for that episode.
- Patients MUST wait at least 4 hours before treating another episode of breakthrough pain with ACTIQ. Once a successful dose has been found (i.e., an average episode is treated with a single unit), patients should limit consumption to four or fewer units per day.
- Dosage adjustment of ACTIQ may be required in some patients in order to continue to provide adequate relief of breakthrough pain.
- Generally, the ACTIQ dose should be increased only when a single administration of the current dose fails to adequately treat the breakthrough pain episode for several consecutive episodes.
- If the patient experiences greater than four breakthrough pain episodes per day, the dose of the maintenance (around-the-clock) opioid used for persistent pain should be re-evaluated.
Administration of ACTIQ
Open the blister package with scissors immediately prior to product use. The patient should place the ACTIQ unit in his or her mouth between the cheek and lower gum, occasionally moving the drug matrix from one side to the other using the handle. The ACTIQ unit should be sucked, not chewed. A unit dose of ACTIQ, if chewed and swallowed, might result in lower peak concentrations and lower bioavailability than when consumed as directed [see Clinical Pharmacology (12.3)].
The ACTIQ unit should be consumed over a 15-minute period. Longer or shorter consumption times may produce less efficacy than reported in ACTIQ clinical trials. If signs of excessive opioid effects appear before the unit is consumed, remove the drug matrix from the patient’s mouth immediately and decrease future doses.
Discontinuation of ACTIQ
When opioid therapy is no longer required, consider discontinuing ACTIQ along with a gradual downward tapering (titration) of other opioids to minimize possible withdrawal effects. In patients who continue to take their chronic opioid therapy for persistent pain but no longer require treatment for breakthrough pain, ACTIQ therapy can usually be discontinued immediately. [see Drug Abuse and Dependence (9.3)]
Disposal of ACTIQ
After consumption of the unit is complete and the matrix is totally dissolved, throw away the handle in a trash container that is out of the reach of children.
- If any of the drug matrix remains on the handle, place the handle under hot running tap water until all of the drug matrix is dissolved, and then dispose of the handle in a place that is out of the reach of children.
- Dispose of handles in the child-resistant container (as described in steps 1 and 2) at least once a day.
If the temporary storage bottle provided as part of the ACTIQ Child Safety Kit is available, partially consumed units may be stored in the specially provided child-resistant container out of the reach of children until proper disposal is possible.
Unopened units remaining from a prescription must be properly disposed as soon as they are no longer needed.
To dispose of the unused ACTIQ units:
- Remove the ACTIQ unit from its blister package using scissors, and hold ACTIQ by its handle over the toilet bowl.
- Using wire-cutting pliers cut off the drug matrix end so that it falls into the toilet.
- Dispose of the handle in a place that is out of the reach of children.
- Repeat steps 1, 2, and 3 for each ACTIQ unit. Flush the toilet twice after 5 units have been cut and deposited into the toilet.
Do not flush the entire ACTIQ units, ACTIQ handles, blister packages, or cartons down the toilet. Dispose of the handle where children cannot reach it.
In the event that a caregiver requires additional assistance in disposing of excess unusable units that remain in the home after a patient has expired, instruct them to call the toll-free number for Teva Pharmaceuticals (1-888-483-8279) or seek assistance from their local DEA office.




