Drug Detail:Altuviiio (Antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl)
Generic Name: EFANESOCTOCOG ALFA 250[iU] in 3mL;
Dosage Form: injection kit
Drug Class: Miscellaneous coagulation modifiers
For intravenous use after reconstitution only.
Dose
- Each ALTUVIIIO vial label states the Factor VIII potency in international units (IU). One IU corresponds to the Factor VIII activity contained in one milliliter of normal human plasma, as defined by the current World Health Organization (WHO) international standard for Factor VIII concentrate.
- Potency assignment for ALTUVIIIO is determined using an activated partial thromboplastin time (aPTT)-based one-stage clotting assay. It is recommended to use a validated one-stage clotting assay to measure ALTUVIIIO Factor VIII activity in plasma. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid based aPTT reagent in one-stage clotting assay by approximately 2.5-fold [see Warnings and Precautions (5.3)].
For the dose of 50 IU/kg, the expected in vivo peak increase in Factor VIII level expressed as IU/dL (or % of normal) is estimated using the following formula:
Estimated Increment of Factor VIII (IU/dL or % of normal) = 50 IU/kg × 2 (IU/dL per IU/kg)
To achieve a specific target Factor VIII activity level, use the following formula: Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL).
Routine Prophylaxis
The recommended dosing for routine prophylaxis for adults and children is 50 IU/kg of ALTUVIIIO administered once weekly.
On-demand Treatment and Control of Bleeding Episodes
ALTUVIIIO dosing for the on-demand treatment and control of bleeding episodes is provided in Table 1.
| Type of Bleeding | Recommended Dose | Additional Information |
|---|---|---|
| Minor and Moderate For example: uncomplicated joint bleeds, minor muscular bleeds, mucosal or subcutaneous bleeds |
Single dose of 50 IU/kg | For minor and moderate bleeding episodes occurring within 2 to 3 days after a prophylactic dose, a lower dose of 30 IU/kg dose may be used. Additional doses of 30 or 50 IU/kg every 2 to 3 days may be considered. |
| Major For example: Intracranial, retroperitoneal, iliopsoas and neck bleeds, muscle bleeds with compartment syndrome and bleeds associated with a significant decrease in the hemoglobin level |
Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days can be considered. |
For resumption of prophylaxis (if applicable) after treatment of a bleed, it is recommended to allow an interval of at least 72 hours between the last 50 IU/kg dose for treatment of a bleed and resuming prophylaxis dosing. Thereafter, prophylaxis can be continued as usual on the patient's regular schedule.
Perioperative Management
ALTUVIIIO dosing for perioperative management is provided in Table 2.
| Type of Surgery | Pre-operative Dose | Post-operative Dose |
|---|---|---|
| Minor For example: Tooth extraction |
Single dose of 50 IU/kg | An additional dose of 30 or 50 IU/kg after 2 to 3 days may be considered. |
| Major For example: Intracranial, intra-abdominal, joint replacement surgery, or complicated dental procedures. |
Single dose of 50 IU/kg | Additional doses of 30 or 50 IU/kg every 2 to 3 days may be administered as clinically needed for perioperative management. |
Preparation and Reconstitution
- Use aseptic technique and a flat work surface during the reconstitution procedure.
- Allow the ALTUVIIIO vial, containing the white to off-white lyophilized powder, and the prefilled diluent syringe to reach room temperature before use.
- Remove the plastic cap from the ALTUVIIIO vial and wipe the rubber stopper of the vial with an alcohol wipe. Allow the rubber stopper to dry.
- Completely remove the backing from the vial adapter package by peeling back the lid. Do not remove the vial adapter from the package or touch the inside of the package of the adapter.
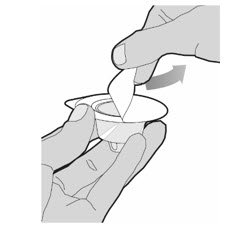
- Keep the vial on a flat surface. Hold the vial with one hand and using the other hand, place the vial adapter in its package over the vial. The spike should be placed directly above the center of the rubber stopper. Push the vial adapter straight down until the spike on the vial adapter punctures the center of the vial stopper and is fully inserted.
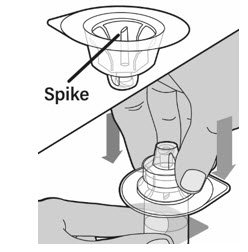
- Lift the package cover away from the vial adapter and throw away the cover.
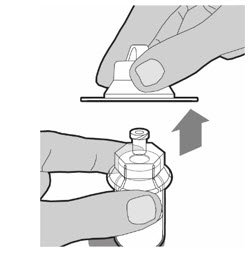
- Only use the prefilled diluent syringe provided to reconstitute the powdered medicine. Hold the plunger rod by the circular disk. Place the tip of the plunger rod into the end of the prefilled diluent syringe. Turn the plunger rod to the right until it is firmly attached.
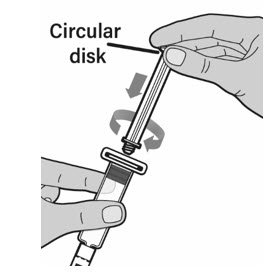
- With one hand, hold the prefilled diluent syringe directly under the cap with the cap pointing up. Make sure you are holding the prefilled diluent syringe by the ridged part directly under the cap. Do not use if the cap has been removed or is not securely attached.
- With your other hand, grasp the cap and bend it at a 90 degree angle until it snaps off. After the cap snaps off, you will see the glass tip of the prefilled diluent syringe. Do not touch the glass tip of the prefilled diluent syringe or the inside of the cap.
- Be sure the vial is sitting on a flat surface. Insert the tip of the prefilled diluent syringe into the vial adapter opening. Turn the prefilled diluent syringe to the right until it is securely attached to the vial adapter.
- Slowly push down on the plunger rod to inject all of the liquid (diluent) from the prefilled diluent syringe into the vial. The plunger rod may rise slightly afterward. This is normal.
- With the prefilled diluent syringe still connected to the adapter, gently swirl the vial until the powder is completely dissolved. Check the solution through the vial to make sure the powder is fully dissolved. The solution should look clear and colorless to opalescent. Do not shake. Do not use the reconstituted ALTUVIIIO if it contains visible particles or is cloudy.
POOLING: pooling is the process of combining two or more reconstituted vials into a larger luer lock syringe (not provided in the carton). If the dose requires more than one vial, reconstitute each vial as described above (See Steps 3–12) with the prefilled diluent syringe provided. Do not detach the prefilled diluent syringe until you are ready to attach the larger luer lock syringe to the next vial. Keep the vial adapter attached to the vial as you will need it for attaching a larger luer lock syringe. Use a larger luer lock plastic syringe to combine the contents of the reconstituted vials into the syringe, similar as described in the Steps 13–14. Repeat this pooling procedure with each vial you will be using. Once you have pooled the required dose, proceed with the administration steps using the larger luer lock syringe. - Make sure the plunger rod is pressed all the way down and the diluent syringe is firmly attached to the vial adapter. Turn the vial upside-down. Slowly pull down on the plunger rod to draw all the solution from the vial into the diluent syringe. Be careful not to pull the plunger rod completely out of the diluent syringe.
- Gently unscrew the diluent syringe from the vial adapter by turning it to the right. Dispose of the vial with the adapter still attached. If you are not ready to inject, put the syringe cap carefully back onto the syringe tip. Do not touch the syringe tip or the inside of the cap.
- Use the reconstituted ALTUVIIIO as soon as possible, but no later than 3 hours after reconstitution. Do not touch the glass tip of the syringe if not used immediately after reconstitution. Protect from direct sunlight. Do not refrigerate after reconstitution.
Administration
For intravenous use only.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use ALTUVIIIO solution if particulate matter or discoloration is observed.
- Do not administer reconstituted ALTUVIIIO in the same tubing or container with other medications.
Administration Steps:
- Attach the syringe to the connector end of the infusion set tubing by turning it to the right until it is securely attached.
- Push the plunger rod until all air is removed from the syringe and ALTUVIIIO has filled the infusion set needle. Do not push ALTUVIIIO solution through the needle.
- Remove the protective needle cover from the infusion set needle.
- Perform intravenous injection. The rate of administration should be determined by the patient's comfort level and no faster than:
- For adults and adolescents: 1–2 minutes per vial.
- For children: 2–3 minutes per vial if body weight is greater than or equal to 20 kg; or 6 minutes per vial if body weight is less than 20 kg.
- After infusing ALTUVIIIO, remove and properly discard the infusion set.








