Drug Detail:Ampyra (Dalfampridine [ dal-fam-pri-deen ])
Generic Name: DALFAMPRIDINE 10mg
Dosage Form: tablet, film coated, extended release
Drug Class: Miscellaneous central nervous system agents
Drug Detail:Ampyra (Dalfampridine [ dal-fam-pri-deen ])
Generic Name: DALFAMPRIDINE 10mg
Dosage Form: tablet, film coated, extended release
Drug Class: Miscellaneous central nervous system agents
The maximum recommended dosage of AMPYRA is one 10 mg tablet twice daily and should not be exceeded. Take doses approximately 12 hours apart.
There is no evidence of additional benefit at doses greater than 10 mg twice daily. Adverse reactions, including seizures, and discontinuations because of adverse reactions were more frequent at higher doses.
AMPYRA can be taken with or without food. Administer tablets whole; do not divide, crush, chew, or dissolve AMPYRA tablets.
If a dose is missed, patients should not take double or extra doses.
Estimated creatinine clearance (CrCl) should be known before initiating treatment with AMPYRA, and monitored at least annually during treatment with AMPYRA. CrCl can be estimated using the following equation (multiply by 0.85 for women):
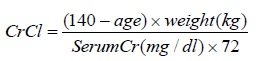
In patients with mild renal impairment (CrCl 51–80 mL/min), AMPYRA plasma levels may approach those seen at a dose of 15 mg twice daily, a dose that is 1.5 times the maximum recommended dose and may be associated with an increased risk of seizures. As mild renal impairment is common after age 50, estimating CrCl is particularly important in these patients. The potential benefits of AMPYRA should be carefully considered against the risk of seizures in these patients [ see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. AMPYRA is contraindicated in patients with moderate or severe renal impairment (CrCl≤50 mL/min).

