Drug Detail:Andexxa (Coagulation factor xa [ koe-ag-ue-lay-tion-fak-tor-xa ])
Generic Name: andexanet alfa 200mg in 20mL
Dosage Form: injection, powder, lyophilized, for solution
Drug Class: Anticoagulant reversal agents
For intravenous (IV) use only.
Dose
There are two dosing regimens (see Table 1). The safety and efficacy of an additional dose have not been established.
| Dose* | Initial IV Bolus | Follow-On IV Infusion | Total Number of 200 mg Vials |
|---|---|---|---|
|
|||
|
Low Dose |
400 mg at a target rate of 30 mg/min |
4 mg/min for up to 120 minutes (480 mg) |
5 |
|
High Dose |
800 mg at a target rate of 30 mg/min |
8 mg/min for up to 120 minutes (960 mg) |
9 |
The recommended dosing of ANDEXXA is based on the specific FXa inhibitor, dose of FXa inhibitor, and time since the patient's last dose of FXa inhibitor (see Table 2).
- Table 2: ANDEXXA Dose Based on Rivaroxaban or Apixaban Dose (Timing of Last Dose of FXa Inhibitor before ANDEXXA Initiation)
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
Reconstitution
- •
- The reconstituted solution contains coagulation factor Xa (recombinant), inactivated-zhzo at a concentration of 10 mg/mL.
- •
- Reconstituted ANDEXXA in vials is stable at room temperature for up to eight hours, or may be stored for up to 24 hours at 2°C to 8°C.
- •
- Reconstituted ANDEXXA in IV bags is stable at room temperature for up to eight hours.
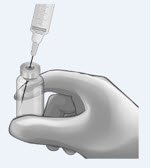
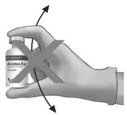
IV Bolus Preparation
Discard the syringe and needle.
Discard the vials, including any unused portion.
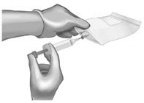
Continuous IV Infusion Preparation
- •
- Follow the same procedure outlined above for IV bolus preparation. Reconstitute the total number of vials needed based on the dose requirements. More than one 40 to 60-mL syringe, or an equivalent 100-mL syringe, may be used for transfer of reconstituted solution to the IV bag.
- •
- Infusion will require a 0.2 or 0.22 micron in-line polyethersulfone or equivalent low protein-binding filter.
Administration
- •
- Upon reconstitution, the parenteral drug product should be inspected visually for particulate matter and discoloration prior to administration.
- •
- Administer ANDEXXA intravenously, using a 0.2 or 0.22 micron in-line polyethersulfone or equivalent low protein-binding filter.
- •
- Start the bolus at a target rate of approximately 30 mg/min.
- •
- Within two minutes following the bolus dose, administer the continuous IV infusion for up to 120 minutes.
Restarting Anticoagulant Therapy
Patients treated with FXa inhibitor therapy have underlying disease states that predispose them to thromboembolic events. Reversing FXa inhibitor therapy exposes patients to the thrombotic risk of their underlying disease. To reduce the risk of thrombosis, resume anticoagulant therapy as soon as medically appropriate following treatment with ANDEXXA.