Drug Detail:Benefix (Coagulation factor ix [ koe-ag-yoo-lay-shun-fak-tor-nine ])
Generic Name: COAGULATION FACTOR IX RECOMBINANT HUMAN 1000[iU] in 5mL; ; ISOPROPYL ALCOHOL 70mL in 100mL
Dosage Form: injection
Drug Class: Miscellaneous coagulation modifiers
For intravenous use after reconstitution only.
- •
- Each vial of BeneFIX has the recombinant Factor IX (rFIX) potency in the International Units (IU) stated on the vial.
- •
- Initiate treatment under the supervision of a physician experienced in the treatment of hemophilia B.
- •
- Dosage and duration of treatment with BeneFIX depend on the severity of the factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of factor IX.
- •
- Dosing of BeneFIX may differ from that of plasma-derived factor IX products [see Clinical Pharmacology (12)]. Subjects at the low end of the observed factor IX recovery may require upward dosage adjustment of BeneFIX to as much as two times (2X) the initial empirically calculated dose in order to achieve the intended rise in circulating factor IX activity.
- •
- Monitor patients using a factor IX activity assay to ensure that the desired factor IX activity level has been achieved. Titrate the dose using the factor IX activity, pharmacokinetic parameters, such as half-life and recovery, as well as taking the clinical situation into consideration in order to adjust the dose as appropriate.
Dose
Calculating Initial Dose
Use the following formula to calculate the initial dose of BeneFIX:
|
number of factor IX IU required (IU) |
= |
body weight (kg) |
× |
desired factor IX increase (% of normal or IU/dL) |
× |
reciprocal of observed recovery (IU/kg per IU/dL) |
Average Recovery
Adolescents/Adults (≥12 years)
In adults, on average, one International Unit (IU) of BeneFIX per kilogram of body weight increased the circulating activity of factor IX by 0.8 ± 0.2 IU/dL (range 0.4 to 1.2 IU/dL). Use the following formula to estimate the dose with 0.8 IU/dL average increase of factor IX per IU/kg body weight administered:
|
number of factor IX IU required (IU) |
= |
body weight (kg) |
× |
desired factor IX increase (% of normal or IU/dL) |
× |
1.3 (IU/kg per IU/dL) |
Children (<12 years)
In children, on average, one international unit of BeneFIX per kilogram of body weight increased the circulating activity of factor IX by 0.7 ± 0.3 IU/dL (range 0.2 to 2.1 IU/dL; median of 0.6 IU/dL per IU/kg). Use the following formula to estimate the dose with 0.7 IU/dL average increase of factor IX per IU/kg body weight administered:
|
number of factor IX IU required (IU) |
= |
body weight (kg) |
× |
desired factor IX increase (% of normal or IU/dL) |
× |
1.4 (IU/kg per IU/dL) |
Doses administered should be titrated to the patient's clinical response. Patients may vary in their pharmacokinetic (e.g., half-life, recovery) and clinical responses to BeneFIX. Although the dose can be estimated by the calculations above, it is highly recommended that, whenever possible, appropriate laboratory tests, including serial factor IX activity assays, be performed.
Dosing for On-demand Treatment and Control of Bleeding Episodes and Perioperative Management
|
Type of Hemorrhage |
Circulating Factor IX Activity Required [% of normal or (IU/dL)] |
Dosing Interval [hours] |
Duration of Therapy [days] |
|
Minor Uncomplicated hemarthroses, superficial muscle, or soft tissue |
20-30 |
12-24 |
1-2 |
|
Moderate Intramuscle or soft tissue with dissection, mucous membranes, dental extractions, or hematuria |
25-50 |
12-24 |
Treat until bleeding stops and healing begins, about 2 to 7 days |
|
Major Pharynx, retropharynx, retroperitoneum, CNS, surgery |
50-100 |
12-24 |
7-10 |
Adapted from: Roberts and Eberst1.
Routine Prophylaxis
For long-term prophylaxis against bleeding, the recommended regimen is 100 IU/kg once weekly. Children (<12 years) have lower recovery, shorter half-life and higher clearance (based on per kg body weight) as compared to adolescents and adults. Adjust the dosing regimen (dose or frequency) based on the patient's clinical response.
Preparation and Reconstitution
The procedures below are provided as general guidelines for the preparation and reconstitution of BeneFIX.
Preparation
- 1.
- Always wash hands before performing the following procedures.
- 2.
- Use aseptic technique (meaning clean and germ-free) during the reconstitution procedure.
- 3.
- Use all components in the reconstitution and administration of this product as soon as possible after opening their sterile containers to minimize unnecessary exposure to the atmosphere.
- 4.
- Pooling: If needing more than one vial of BeneFIX per infusion, reconstitute each vial according to the following instructions. Remove the diluent syringe leaving the vial adapter in place, and use a separate large luer lock syringe to draw back the reconstituted contents of each vial. Do not detach the diluent syringes or the large luer lock syringe until you are ready to attach the large luer lock syringe to the next vial adapter.
Reconstitution
- 1.
- If refrigerated, allow the vial of lyophilized BeneFIX and the pre-filled diluent syringe to reach room temperature.
- 2.
- Remove the plastic flip-top cap from the BeneFIX vial to expose the central portions of the rubber stopper.
- 3.
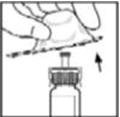
- Wipe the top of the vial with the alcohol swab provided, or use another antiseptic solution, and allow to dry. After cleaning, do not touch the rubber stopper with your hand or allow it to touch any surface.
- 4.
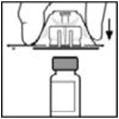
- Peel back the cover from the clear plastic vial adapter package. Do not remove the adapter from the package.
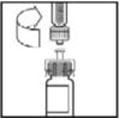
- 5.
- Place the vial on a flat surface. While holding the adapter in the package, place the vial adapter over the vial and press down firmly on the package until the adapter spike penetrates the vial stopper.
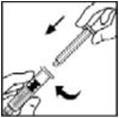
- 6.
- Grasp the plunger rod as shown in the diagram. Avoid contact with the shaft of the plunger rod. Attach the threaded end of the plunger rod to the diluent syringe plunger by pushing and turning firmly.
- 7.
- Break off the tamper-resistant plastic-tip cap from the diluent syringe by snapping the perforation of the cap. Do not touch the inside of the cap or the syringe tip. The diluent syringe may need to be recapped (if not administering reconstituted BeneFIX immediately), so place the cap on its top on a clean surface in a spot where it would be least likely to become environmentally contaminated.
- 8.
- Lift the package away from the adapter and discard the package.
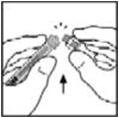
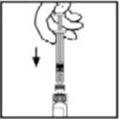
- 9.
- Place the vial on a flat surface. Connect the diluent syringe to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
- 10.
- Slowly depress the plunger rod to inject all the diluent into the BeneFIX vial.
- 11.
- Without removing the syringe, gently swirl the contents of the vial until the powder is dissolved.
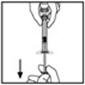
- 12.
- Invert the vial and slowly draw the solution into the syringe.
- 13.
- Detach the syringe from the vial adapter by gently pulling and turning the syringe counter-clockwise. Discard the vial with the adapter attached.
- 14.
- The reconstituted solution should be clear and colorless. If it is not, discard and use a new kit. If the solution is not to be used immediately, recap the syringe. Do not touch the syringe tip or the inside of the cap.
- 15.
- Store the reconstituted solution at room temperature and use it within 3 hours.
Note: BeneFIX, when reconstituted, contains polysorbate-80, which is known to increase the rate of di-(2-ethylhexyl) phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of BeneFIX, including storage time elapsed in a PVC container following reconstitution. It is important that the recommendations for dosage and administration be followed closely [see Dosage and Administration (2.1, 2.3)].
Note: The tubing of the infusion set included with this kit does not contain DEHP.
Administration
For intravenous use after reconstitution only.
The safety and efficacy of administration by continuous infusion have not been established [see Warnings and Precautions (5.2)].
- •
- Inspect BeneFIX solution for particulate matter and discoloration prior to administration, whenever solution and container permit.
- •
- Administer BeneFIX using the tubing provided in this kit, and the pre-filled diluent syringe provided, or a single sterile disposable plastic syringe.
- •
- Do not mix or administer BeneFIX in the same tubing or container with other medicinal products.
Administration
- 1.
- Attach the syringe to the luer end of the infusion set tubing provided.
- 2.
- Apply a tourniquet and prepare the injections site by wiping the skin well with an alcohol swab provided in the kit.
- 3.
- Perform venipuncture. Insert the needle on the infusion set tubing into the vein, and remove the tourniquet. Inject the reconstituted BeneFIX intravenously over several minutes. Adjust the rate of administration based on the patient's comfort level.
Note: Agglutination of red blood cells in the tubing/syringe has been reported with the administration of BeneFIX. No adverse events have been reported in association with this observation. To minimize the possibility of agglutination, it is important to limit the amount of blood entering the tubing. Blood should not enter the syringe. If red blood cell agglutination is observed in the tubing or syringe, discard all material (tubing, syringe and BeneFIX solution) and resume administration with a new package. - 4.
- Following completion of BeneFIX treatment, remove and discard the infusion set. Dispose of all unused solution, empty vial(s), and used needles and syringes in an appropriate container.