Drug Detail:Berinert (Complement c1 esterase inhibitor [ kom-ple-ment-c1 es-ter-ase-in-hib-it-or ])
Generic Name: HUMAN C1-ESTERASE INHIBITOR 500[iU] in 10mL;
Dosage Form: injection
Drug Class: Hereditary angioedema agents
For intravenous use only.
Administer BERINERT at a dose of 20 International Units (IU) per kg body weight by intravenous injection. Doses lower than 20 IU/kg body weight should not be administered.
BERINERT is provided as a freeze-dried powder for reconstitution with the Sterile Water for Injection, USP provided. Store the vial in the original carton in order to protect from light. Do not freeze.
Preparation and Handling
- Check the expiration date on the product vial label and on the administration kit. Do not use any component that is beyond the expiration date. Do not use if any components are damaged or opened.
- Prepare and administer using aseptic techniques [see Dosage and Administration (2.2)].
- Use the silicone-free syringe, provided, for administration of BERINERT [see How Supplied/Storage and Handling (16)].
- After reconstitution and prior to administration, inspect BERINERT visually for particulate matter and discoloration. The reconstituted solution should be colorless, clear, and free from visible particles. Do not use if the solution is cloudy, discolored, or contains particulates.
- The BERINERT vial is for single use only. BERINERT contains no preservative. Any product that has been reconstituted should be used promptly. The reconstituted solution must be used within 8 hours. Discard partially used vials.
- Do not freeze the reconstituted solution.
Reconstitution and Administration
Each BERINERT vial containing 500 IU of C1 esterase inhibitor as a lyophilized concentrate for reconstitution with 10 mL of Sterile Water for Injection, USP provided.
Use either the Mix2Vial® transfer set provided with BERINERT [see How Supplied/Storage and Handling (16.1)] or a commercially available double-ended needle and vented filter spike.
Reconstitution
The procedures below are provided as general guidelines for the reconstitution and administration of BERINERT.
- Ensure that the BERINERT vial and diluent vial are at room temperature.
- Place the BERINERT vial, diluent vial and Mix2Vial transfer set on a flat surface.
- Remove the flip caps from the BERINERT and diluent vials. Wipe the vial stoppers with the alcohol swab provided. Allow to dry prior to opening the Mix2Vial transfer set package.
- Open the Mix2Vial transfer set package by peeling away the lid (Figure 1). Leave the Mix2Vial transfer set in the clear package.
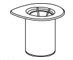
Figure 1
- Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Figure 2).
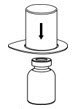
Figure 2
- Carefully remove the clear package from the Mix2Vial transfer set. Make sure that you pull up only the clear package, and not the Mix2Vial transfer set (Figure 3).
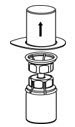
Figure 3
- With the BERINERT vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the BERINERT vial (Figure 4). The diluent will automatically transfer into the BERINERT vial.
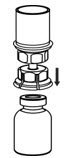
Figure 4
- With the diluent and BERINERT vial still attached to the Mix2Vial transfer set, gently swirl the BERINERT vial to ensure that the BERINERT is fully dissolved (Figure 5). Do not shake the vial.
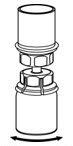
Figure 5
- With one hand, grasp the BERINERT-side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set and unscrew (counter clockwise) the set into two pieces (Figure 6).
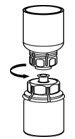
Figure 6
- Carefully look at reconstituted solution in each vial of BERINERT. It should be colorless, clear, and free from visible particles. Do not use the vial if the liquid looks cloudy, contains particles, or has changed color. Do not use if the expiration date on the label has expired.
- Draw air into the empty, sterile, silicone-free syringe provided. While the BERINERT vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the BERINERT vial. While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Figure 7). If BERINERT is not immediately used, please store reconstituted solution in the vial.
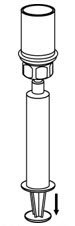
Figure 7
- Now that the concentrate has been transferred into the syringe, firmly grasp the barrel of the syringe (keeping the plunger facing down) and unscrew (counter clockwise) the syringe from the Mix2Vial transfer set (Figure 8). Attach the syringe to a suitable intravenous administration set.
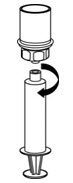
Figure 8
- If patient requires more than one vial, a new unused Mix2Vial transfer set and a new silicone-free syringe should be used for each BERINERT vial.
- Do not refrigerate after reconstitution. When reconstitution is carried out using aseptic technique, administration may begin within 8 hours, provided the solution has been stored at up to 30°C (86°F). Do not refrigerate or freeze the reconstituted solution. Only store the reconstituted solution in the vial.
- Do not mix BERINERT with other medicinal products. Administer BERINERT by a separate infusion line.
- Use aseptic technique when administering BERINERT.
- Use the silicone-free syringe provided in the administration kit [see How Supplied/Storage and Handling (16)].
- Follow recommended venipuncture guidelines for initiating intravenous therapy.
- Administer BERINERT by slow intravenous injection at a rate of approximately 4 mL per minute. Please refer to the illustration in step 6 of the self-administration section in the Patient Product Information (PPI) section.
- For self-administration, provide the patient with instructions and training for intravenous injection outside of a clinic setting so patients may self-administer BERINERT upon recognition of symptoms of an HAE attack [see Patient Counseling Information (17)].
- After administration, immediately discard any unused product and all used disposable supplies in accordance with local requirements.












