Drug Detail:Botox (Onabotulinumtoxina [ on-a-bot-ue-lye-num-tox-in-a ])
Generic Name: BOTULINUM TOXIN TYPE A 100[USP'U]
Dosage Form: injection, powder, lyophilized, for solution
Drug Class: Skeletal muscle relaxants
2.1 Instructions for Safe Use
The potency Units of BOTOX (onabotulinumtoxinA) for injection are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method [see Warnings and Precautions (5.2) and Description (11)].
Indication specific dosage and administration recommendations should be followed. When initiating treatment, the lowest recommended dose should be used. In treating adult patients for one or more indications, the maximum cumulative dose should not exceed 400 Units, in a 3-month interval. In pediatric patients, the total dose should not exceed the lower of 10 Units/kg body weight or 340 Units, in a 3-month interval [see Dosage and Administration (2.7)].
The safe and effective use of BOTOX depends upon proper storage of the product, selection of the correct dose, and proper reconstitution and administration techniques. An understanding of standard electromyographic techniques is also required for treatment of strabismus, upper or lower limb spasticity, and may be useful for the treatment of cervical dystonia. Physicians administering BOTOX must understand the relevant neuromuscular and structural anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures and disease, especially when injecting near the lungs.
Do not use BOTOX and contact Allergan (1-800-890-4345) if:
- the carton labeling does not contain an intact seal with a translucent silver Allergan logo (on both ends of the carton) or the seal has a black circle with a diagonal line through it (i.e., prohibition sign),
- the vial label does not contain a holographic film containing the name “Allergan” within rainbow colored horizontal lines, or
- the U.S. License number 1145 is not present on the vial label and carton labeling [see How Supplied/Storage and Handling (16)].
2.2 Preparation and Dilution Technique
Prior to injection, reconstitute each vacuum-dried vial of BOTOX with only sterile, preservative-free 0.9% Sodium Chloride Injection, USP. Draw up the proper amount of diluent in the appropriate size syringe (see Table 1, or for specific instructions for detrusor overactivity associated with a neurologic condition, see Section 2.3), and slowly inject the diluent into the vial. Discard the vial if a vacuum does not pull the diluent into the vial. Gently mix BOTOX with the diluent by rotating the vial. Record the date and time of reconstitution on the space on the label. BOTOX should be administered within 24 hours after reconstitution. During this time period, unused reconstituted BOTOX should be stored in a refrigerator (2° to 8°C) for up to 24 hours until time of use. BOTOX vials are for single-dose only. Discard any unused portion.
| Diluent* Added to 100 Unit Vial | Resulting Dose Units per 0.1 mL | Diluent* Added to 200 Unit Vial | Resulting Dose Units per 0.1 mL |
| 1 mL 2 mL 4 mL 8 mL 10 mL |
10 Units 5 Units 2.5 Units 1.25 Units 1 Unit |
1 mL 2 mL 4 mL 8 mL 10 mL |
20 Units 10 Units 5 Units 2.5 Units 2 Units |
*Preservative-free 0.9% Sodium Chloride Injection, USP Only
**For Detrusor Overactivity associated with a Neurologic Condition Dilution, see Section 2.3
Note: These dilutions are calculated for an injection volume of 0.1 mL. A decrease or increase in the BOTOX dose is also possible by administering a smaller or larger injection volume - from 0.05 mL (50% decrease in dose) to 0.15 mL (50% increase in dose).
An injection of BOTOX is prepared by drawing into an appropriately sized sterile syringe an amount of the properly reconstituted toxin slightly greater than the intended dose. Air bubbles in the syringe barrel are expelled and the syringe is attached to an appropriate injection needle. Patency of the needle should be confirmed. A new, sterile needle and syringe should be used to enter the vial on each occasion for removal of BOTOX.
Reconstituted BOTOX should be clear, colorless, and free of particulate matter. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and whenever the solution and the container permit.
2.3 Adult Bladder Dysfunction
General
Patients must not have a urinary tract infection (UTI) at the time of treatment. Prophylactic antibiotics, except aminoglycosides, [see Drug Interactions (7.1)] should be administered 1-3 days pre-treatment, on the treatment day, and 1-3 days post-treatment to reduce the likelihood of procedure-related UTI.
Patients should discontinue anti-platelet therapy at least 3 days before the injection procedure. Patients on anti-coagulant therapy need to be managed appropriately to decrease the risk of bleeding.
Appropriate caution should be exercised when performing a cystoscopy.
Overactive Bladder
An intravesical instillation of diluted local anesthetic with or without sedation may be used prior to injection, per local site practice. If a local anesthetic instillation is performed, the bladder should be drained and irrigated with sterile saline before injection.
The recommended dose is 100 Units of BOTOX, and is the maximum recommended dose. The recommended dilution is 100 Units/10 mL with preservative-free 0.9% Sodium Chloride Injection, USP (see Table 1). Dispose of any unused saline.
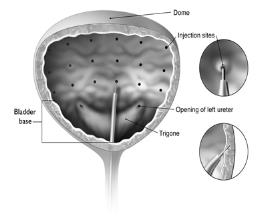
Reconstituted BOTOX (100 Units/10 mL) is injected into the detrusor muscle via a flexible or rigid cystoscope, avoiding the trigone. The bladder should be instilled with enough saline to achieve adequate visualization for the injections, but over-distension should be avoided.
The injection needle should be filled (primed) with approximately 1 mL of reconstituted BOTOX prior to the start of injections (depending on the needle length) to remove any air.
The needle should be inserted approximately 2 mm into the detrusor, and 20 injections of 0.5 mL each (total volume of 10 mL) should be spaced approximately 1 cm apart (see Figure 1). For the final injection, approximately 1 mL of sterile normal saline should be injected so that the remaining BOTOX in the needle is delivered to the bladder. After the injections are given, patients should demonstrate their ability to void prior to leaving the clinic. The patient should be observed for at least 30 minutes post-injection and until a spontaneous void has occurred.
Patients should be considered for reinjection when the clinical effect of the previous injection has diminished (median time until patients qualified for the second treatment of BOTOX in double-blind, placebo-controlled clinical studies was 169 days [~24 weeks]), but no sooner than 12 weeks from the prior bladder injection.
Figure 1: Injection Pattern for Intradetrusor Injections for Treatment of Overactive Bladder and Detrusor Overactivity Associated with a Neurologic Condition
Detrusor Overactivity associated with a Neurologic Condition
An intravesical instillation of diluted local anesthetic with or without sedation, or general anesthesia may be used prior to injection, per local site practice. If a local anesthetic instillation is performed, the bladder should be drained and irrigated with sterile saline before injection.
The recommended dose is 200 Units of BOTOX per treatment, and should not be exceeded.
200 Unit Vial of BOTOX
- Reconstitute a 200 Unit vial of BOTOX with 6 mL of preservative-free 0.9% Sodium Chloride Injection, USP and mix the vial gently.
- Draw 2 mL from the vial into each of three 10 mL syringes.
- Complete the reconstitution by adding 8 mL of preservative-free 0.9% Sodium Chloride Injection, USP into each of the 10 mL syringes, and mix gently. This will result in three 10 mL syringes each containing 10 mL (~67 Units in each), for a total of 200 Units of reconstituted BOTOX.
- Use immediately after reconstitution in the syringe. Dispose of any unused saline.
100 Unit Vial of BOTOX
- Reconstitute two 100 Unit vials of BOTOX, each with 6 mL of preservative-free 0.9% Sodium Chloride Injection, USP and mix the vials gently.
- Draw 4 mL from each vial into each of two 10 mL syringes. Draw the remaining 2 mL from each vial into a third 10 mL syringe for a total of 4 mL in each syringe.
- Complete the reconstitution by adding 6 mL of preservative-free 0.9% Sodium Chloride Injection, USP into each of the 10 mL syringes, and mix gently. This will result in three 10 mL syringes each containing 10 mL (~67 Units in each), for a total of 200 Units of reconstituted BOTOX.
- Use immediately after reconstitution in the syringe. Dispose of any unused saline.
Reconstituted BOTOX (200 Units/30 mL) is injected into the detrusor muscle via a flexible or rigid cystoscope, avoiding the trigone. The bladder should be instilled with enough saline to achieve adequate visualization for the injections, but over-distension should be avoided.
The injection needle should be filled (primed) with approximately 1 mL of reconstituted BOTOX prior to the start of injections (depending on the needle length) to remove any air.
The needle should be inserted approximately 2 mm into the detrusor, and 30 injections of 1 mL (~6.7 Units) each (total volume of 30 mL) should be spaced approximately 1 cm apart (see Figure 1). For the final injection, approximately 1 mL of sterile normal saline should be injected so that the remaining BOTOX in the needle is delivered to the bladder. After the injections are given, the saline used for bladder wall visualization should be drained. The patient should be observed for at least 30 minutes post-injection.
Patients should be considered for re-injection when the clinical effect of the previous injection diminishes (median time to qualification for re-treatment in the double-blind, placebo-controlled clinical studies was 295-337 days [42-48 weeks] for BOTOX 200 Units), but no sooner than 12 weeks from the prior bladder injection.
2.4 Pediatric Detrusor Overactivity associated with a Neurologic Condition
Patients must not have a urinary tract infection (UTI) at the time of treatment. Oral prophylactic antibiotics, except aminoglycosides, [see Drug Interactions (7.1)] should be administered 1-3 days pre-treatment, on the treatment day, and 1-3 days post-treatment to reduce the likelihood of procedure-related UTI. Alternatively, for patients receiving general anesthesia (or conscious sedation) for the treatment of detrusor overactivity associated with a neurologic condition, one dose of IV prophylactic antibiotics, except aminoglycosides, [see Drug Interactions (7.1)] may be administered prior to treatment administration on the day of treatment.
Patients should discontinue anti-platelet therapy at least 3 days before the injection procedure. Patients on anti-coagulant therapy need to be managed appropriately to decrease the risk of bleeding.
Appropriate caution should be exercised when performing a cystoscopy.
- In patients 5 years to less than 12 years of age: Consider general anesthesia (or conscious sedation) prior to injection, per local site practice.
- In patients 12 years of age or older: Consider an intravesical instillation of diluted local anesthetic with or without sedation, or general anesthesia prior to injection, per local site practice.
At a minimum, consider a diluted instillation of local anesthetic for all age groups. If a local anesthetic instillation is performed, drain and irrigate the bladder with sterile saline before injection.
If patient’s body weight is greater than or equal to 34 kg, the recommended dosage is 200 Units of BOTOX per treatment administered as an intradetrusor injection after dilution:
- Reconstitute BOTOX to result in 20 Units BOTOX/mL in the vial(s):
◦ BOTOX 200 Unit vial: add 10 mL of preservative-free 0.9% Sodium Chloride Injection, USP and mix the vial gently.
◦ BOTOX 100 Unit vials: add 5 mL of preservative-free 0.9% Sodium Chloride Injection, USP to each of two 100 Unit vials of BOTOX and mix the vials gently. - Draw 10 mL from the vial(s) into one 10 mL dosing syringe.
- Use immediately after reconstitution in the syringe. Dispose of any unused saline.
If patient’s body weight is less than 34 kg, the recommended dosage is 6 Units/kg body weight administered as a bladder injection after dilution (refer to Table 2):
- Reconstitute BOTOX to result in 20 Units BOTOX/mL in the vial(s):
◦ BOTOX 200 Unit vial: add 10 mL of preservative-free 0.9% Sodium Chloride Injection, USP and mix the vial gently.
◦ BOTOX 100 Unit vial(s): add 5 mL of preservative-free 0.9% Sodium Chloride Injection, USP to one 100 Unit vial of BOTOX (if final dose is less than or equal to 100 U) or to each of two 100 Unit vials of BOTOX (if final dose is greater than 100 U) and mix the vial(s) gently. - Refer to Table 2 for dilution instructions (i.e., the amount of reconstituted BOTOX and additional diluent to draw into one 10 mL dosing syringe).
- Use BOTOX immediately after reconstitution in the syringe. Dispose of any unused preservative-free 0.9% Sodium Chloride Injection, USP.
| Body Weight (kg) |
Volume of reconstituted BOTOX and Diluent* (mL) to draw into dosing syringe to achieve a final volume of 10 mL | Final dose of BOTOX in dosing syringe | |
| BOTOX (mL) |
Diluent* (mL) |
||
| 12 to less than 14 | 3.6 | 6.4 | 72 Units |
| 14 to less than 16 | 4.2 | 5.8 | 84 Units |
| 16 to less than 18 | 4.8 | 5.2 | 96 Units |
| 18 to less than 20 | 5.4 | 4.6 | 108 Units |
| 20 to less than 22 | 6 | 4 | 120 Units |
| 22 to less than 24 | 6.6 | 3.4 | 132 Units |
| 24 to less than 26 | 7.2 | 2.8 | 144 Units |
| 26 to less than 28 | 7.8 | 2.2 | 156 Units |
| 28 to less than 30 | 8.4 | 1.6 | 168 Units |
| 30 to less than 32 | 9 | 1 | 180 Units |
| 32 to less than 34 | 9.6 | 0.4 | 192 Units |
*Preservative-free 0.9% Sodium Chloride Injection, USP Only
Reconstituted BOTOX is injected into the detrusor muscle via a flexible or rigid cystoscope, avoiding the trigone. The bladder should be instilled with enough saline to achieve adequate visualization for the injections, but over-distension should be avoided.
The injection needle should be filled (primed) with approximately 1 mL of reconstituted BOTOX prior to the start of injections (depending on the needle length) to remove any air.
The needle should be inserted approximately 2 mm into the detrusor, and 20 injections of 0.5 mL each (total volume of 10 mL) should be spaced approximately 1 cm apart (see Figure 1). For the final injection, approximately 1 mL of sterile normal saline should be injected so that the remaining BOTOX in the needle is delivered to the bladder. After the injections are given, the saline used for bladder wall visualization should be drained. The patient should be observed for at least 30 minutes post-injection.
Patients should be considered for re-injection when the clinical effect of the previous injection diminishes (median time to qualification for re-treatment in the double-blind, parallel group clinical study was 207 days [30 weeks] for BOTOX 200 Units), but no sooner than 12 weeks from the prior bladder injection.
2.5 Chronic Migraine
The recommended dilution is 200 Units/4 mL or 100 Units/2 mL, with a final concentration of 5 Units per 0.1 mL (see Table 1). The recommended dose for treating chronic migraine is 155 Units administered intramuscularly using a sterile 30-gauge, 0.5 inch needle as 0.1 mL (5 Units) injections per each site. Injections should be divided across 7 specific head/neck muscle areas as specified in the diagrams and Table 3 below. A one inch needle may be needed in the neck region for patients with thick neck muscles. With the exception of the procerus muscle, which should be injected at one site (midline), all muscles should be injected bilaterally with half the number of injection sites administered to the left, and half to the right side of the head and neck. The recommended re-treatment schedule is every 12 weeks.
Diagrams 1-4: Recommended Injection Sites (A through G) for Chronic Migraine
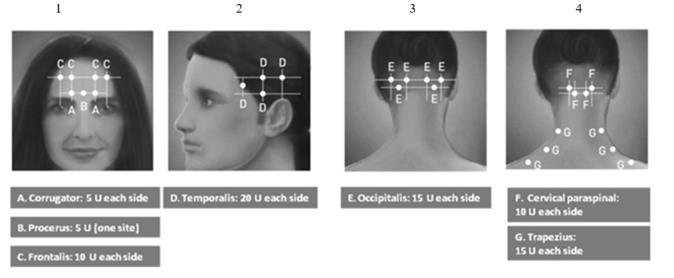
| Head/Neck Area | Recommended Dose (Number of Sitesa) |
| Frontalisb | 20 Units divided in 4 sites |
| Corrugatorb | 10 Units divided in 2 sites |
| Procerus | 5 Units in 1 site |
| Occipitalisb | 30 Units divided in 6 sites |
| Temporalisb | 40 Units divided in 8 sites |
| Trapeziusb | 30 Units divided in 6 sites |
| Cervical Paraspinal Muscle Groupb |
20 Units divided in 4 sites |
| Total Dose: | 155 Units divided in 31 sites |
a Each IM injection site = 0.1 mL = 5 Units BOTOX
b Dose distributed bilaterally
2.6 Adult Spasticity
General
Dosing in initial and sequential treatment sessions should be tailored to the individual based on the size, number and location of muscles involved, severity of spasticity, the presence of local muscle weakness, the patient’s response to previous treatment, or adverse event history with BOTOX.
The recommended dilution is 200 Units/4 mL or 100 Units/2 mL with preservative-free 0.9% Sodium Chloride Injection, USP (see Table 1). The lowest recommended starting dose should be used, and no more than 50 Units per site should generally be administered. An appropriately sized needle (e.g., 25-30 gauge) may be used for superficial muscles, and a longer 22 gauge needle may be used for deeper musculature. Localization of the involved muscles with techniques such as needle electromyographic guidance, nerve stimulation, or ultrasound is recommended.
Repeat BOTOX treatment may be administered when the effect of a previous injection has diminished, but generally no sooner than 12 weeks after the previous injection. The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of BOTOX and muscles to be injected.
Adult Upper Limb Spasticity
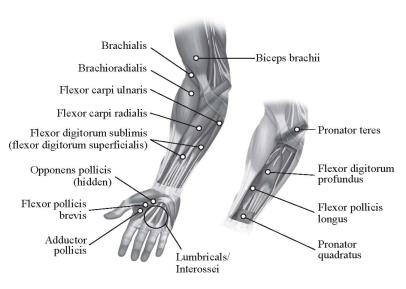
In clinical trials, doses ranging from 75 Units to 400 Units were divided among selected muscles (see Table 4 and Figure 2) at a given treatment session.
| Muscle | Recommended Dose Total Dosage (Number of Sites) |
| Biceps Brachii | 60 Units to 200 Units divided in 2 to 4 sites |
| Brachioradialis | 45 Units to 75 Units divided in 1 to 2 sites |
| Brachialis | 30 Units to 50 Units divided in 1 to 2 sites |
| Pronator Teres | 15 Units to 25 Units in 1 site |
| Pronator Quadratus | 10 Units to 50 Units in 1 site |
| Flexor Carpi Radialis | 12.5 Units to 50 Units in 1 site |
| Flexor Carpi Ulnaris | 12.5 Units to 50 Units in 1 site |
| Flexor Digitorum Profundus | 30 Units to 50 Units in 1 site |
| Flexor Digitorum Sublimis | 30 Units to 50 Units in 1 site |
| Lumbricals/Interossei | 5 Units to 10 Units in 1 site |
| Adductor Pollicis | 20 Units in 1 site |
| Flexor Pollicis Longus | 20 Units in 1 site |
| Flexor pollicis brevis/ Opponens pollicis | 5 Units to 25 Units in 1 site |
Figure 2: Injection Sites for Adult Upper Limb Spasticity
Adult Lower Limb Spasticity
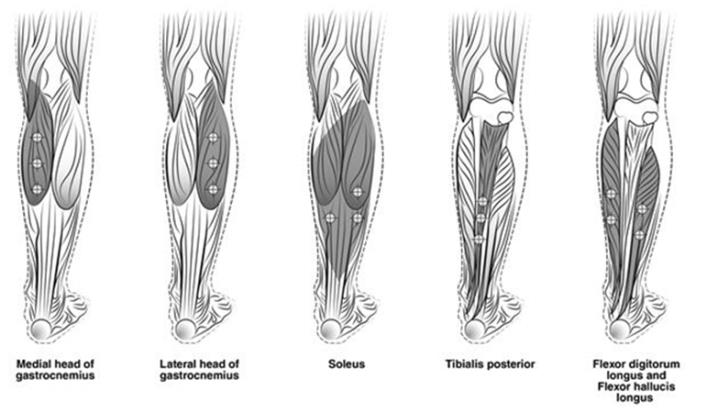
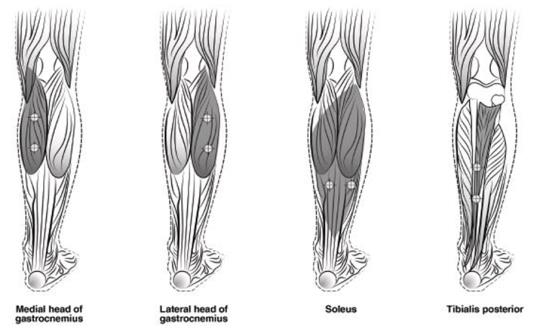
The recommended dose for treating adult lower limb spasticity is 300 Units to 400 Units divided among 5 muscles (gastrocnemius, soleus, tibialis posterior, flexor hallucis longus and flexor digitorum longus) (see Table 5 and Figure 3).
| Muscle | Recommended Dose Total Dosage (Number of Sites) |
| Gastrocnemius medial head | 75 Units divided in 3 sites |
| Gastrocnemius lateral head | 75 Units divided in 3 sites |
| Soleus | 75 Units divided in 3 sites |
| Tibialis Posterior | 75 Units divided in 3 sites |
| Flexor hallucis longus | 50 Units divided in 2 sites |
| Flexor digitorum longus | 50 Units divided in 2 sites |
Figure 3: Injection Sites for Adult Lower Limb Spasticity
2.7 Pediatric Spasticity
General
Localization of the involved muscles with techniques such as needle electromyographic guidance, nerve stimulation, or ultrasound is recommended. When treating both lower limbs or the upper and lower limbs in combination, the total dose should not exceed the lower of 10 Units/kg body weight or 340 Units, in a 3-month interval [see Boxed Warning and Warnings and Precautions (5.1, 5.6)]. Additional general adult spasticity dosing information is also applicable to pediatric spasticity patients [see Dosage and Administration (2.6)].
Pediatric Upper Limb Spasticity
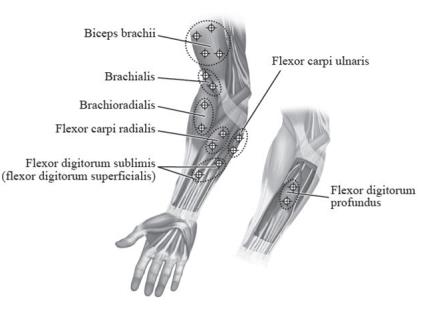
The recommended dose for treating pediatric upper limb spasticity is 3 Units/kg to 6 Units/kg divided among the affected muscles (see Table 6 and Figure 4). The total dose of BOTOX administered per treatment session in the upper limb should not exceed 6 Units/kg or 200 Units, whichever is lower.
| Muscle | Recommended Dose and Number of Sites |
| Biceps Brachii | 1.5 Units/kg to 3 Units/kg divided in 4 sites |
| Brachialis | 1 Unit/kg to 2 Units/kg divided in 2 sites |
| Brachioradialis | 0.5 Units/kg to 1 Unit/kg divided in 2 sites |
| Flexor Carpi Radialis | 1 Unit/kg to 2 Units/kg divided in 2 sites |
| Flexor Carpi Ulnaris | 1 Unit/kg to 2 Units/kg divided in 2 sites |
| Flexor Digitorum Profundus | 0.5 Units/kg to 1 Unit/kg divided in 2 sites |
| Flexor Digitorum Sublimis | 0.5 Units/kg to 1 Unit/kg divided in 2 sites |
Figure 4: Injection Sites for Pediatric Upper Limb Spasticity
Pediatric Lower Limb Spasticity
The recommended dose for treating pediatric lower limb spasticity is 4 Units/kg to 8 Units/kg divided among the affected muscles (see Table 7 and Figure 5). The total dose of BOTOX administered per treatment session in the lower limb should not exceed 8 Units/kg or 300 Units, whichever is lower.
| Muscle | Recommended Dose Total Dosage (Number of Sites) |
| Gastrocnemius medial head | 1 Unit/kg to 2 Units/kg divided in 2 sites |
| Gastrocnemius lateral head | 1 Unit/kg to 2 Units/kg divided in 2 sites |
| Soleus | 1 Unit/kg to 2 Units/kg divided in 2 sites |
| Tibialis Posterior | 1 Unit/kg to 2 Units/kg divided in 2 sites |
Figure 5: Injection Sites for Pediatric Lower Limb Spasticity
2.8 Cervical Dystonia
A double-blind, placebo-controlled study enrolled patients who had extended histories of receiving and tolerating BOTOX injections, with prior individualized adjustment of dose. The mean BOTOX dose administered to patients in this study was 236 Units (25th to 75th percentile range of 198 Units to 300 Units). The BOTOX dose was divided among the affected muscles [see Clinical Studies (14.7)].
Dosing in initial and sequential treatment sessions should be tailored to the individual patient based on the patient’s head and neck position, localization of pain, muscle hypertrophy, patient response, and adverse event history. The initial dose for a patient without prior use of BOTOX should be at a lower dose, with subsequent dosing adjusted based on individual response. Limiting the total dose injected into the sternocleidomastoid muscle to 100 Units or less may decrease the occurrence of dysphagia [see Warnings and Precautions (5.1, 5.5, 5.6)].
The recommended dilution is 200 Units/2 mL, 200 Units/4 mL, 100 Units/1 mL, or 100 Units/2 mL with preservative-free 0.9% Sodium Chloride Injection, USP, depending on volume and number of injection sites desired to achieve treatment objectives (see Table 1). In general, no more than 50 Units per site should be administered using a sterile needle (e.g., 25-30 gauge) of an appropriate length. Localization of the involved muscles with electromyographic guidance may be useful.
Clinical improvement generally begins within the first two weeks after injection with maximum clinical benefit at approximately six weeks post-injection. In the double-blind, placebo-controlled study most subjects were observed to have returned to pre-treatment status by 3 months post-treatment.
2.9 Primary Axillary Hyperhidrosis
The recommended dose is 50 Units per axilla. The hyperhidrotic area to be injected should be defined using standard staining techniques, e.g., Minor’s Iodine-Starch Test. The recommended dilution is 100 Units/4 mL with preservative-free 0.9% Sodium Chloride Injection, USP (see Table 1). Using a sterile 30 gauge needle, 50 Units of BOTOX (2 mL) is injected intradermally in 0.1 to 0.2 mL aliquots to each axilla evenly distributed in multiple sites (10-15) approximately 1-2 cm apart.
Repeat injections for hyperhidrosis should be administered when the clinical effect of a previous injection diminishes.
Instructions for the Minor’s Iodine-Starch Test Procedure:
Patients should shave underarms and abstain from use of over-the-counter deodorants or antiperspirants for 24 hours prior to the test. Patient should be resting comfortably without exercise or hot drinks for approximately 30 minutes prior to the test. Dry the underarm area and then immediately paint it with iodine solution. Allow the area to dry, then lightly sprinkle the area with starch powder. Gently blow off any excess starch powder. The hyperhidrotic area will develop a deep blue-black color over approximately 10 minutes.
Each injection site has a ring of effect of up to approximately 2 cm in diameter. To minimize the area of no effect, the injection sites should be evenly spaced as shown in Figure 6.
Figure 6: Injection Pattern for Primary Axillary Hyperhidrosis
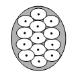
Each dose is injected to a depth of approximately 2 mm and at a 45° angle to the skin surface, with the bevel side up to minimize leakage and to ensure the injections remain intradermal. If injection sites are marked in ink, do not inject BOTOX directly through the ink mark to avoid a permanent tattoo effect.
2.10 Blepharospasm
For blepharospasm, reconstituted BOTOX is injected using a sterile, 27-30 gauge needle without electromyographic guidance. The initial recommended dose is 1.25 Units-2.5 Units (0.05 mL to 0.1 mL volume at each site) injected into the medial and lateral pre-tarsal orbicularis oculi of the upper lid and into the lateral pre-tarsal orbicularis oculi of the lower lid. Avoiding injection near the levator palpebrae superioris may reduce the complication of ptosis. Avoiding medial lower lid injections, and thereby reducing diffusion into the inferior oblique, may reduce the complication of diplopia. Ecchymosis occurs easily in the soft eyelid tissues. This can be prevented by applying pressure at the injection site immediately after the injection.
The recommended dilution to achieve 1.25 Units is 100 Units/8 mL; for 2.5 Units it is 100 Units/4 mL (see Table 1).
In general, the initial effect of the injections is seen within three days and reaches a peak at one to two weeks post-treatment. Each treatment lasts approximately three months, following which the procedure can be repeated. At repeat treatment sessions, the dose may be increased up to two-fold if the response from the initial treatment is considered insufficient, usually defined as an effect that does not last longer than two months. However, there appears to be little benefit obtainable from injecting more than 5 Units per site. Some tolerance may be found when BOTOX is used in treating blepharospasm if treatments are given any more frequently than every three months, and is rare to have the effect be permanent.
The cumulative dose of BOTOX treatment for blepharospasm in a 30-day period should not exceed 200 Units.
2.11 Strabismus
BOTOX is intended for injection into extraocular muscles utilizing the electrical activity recorded from the tip of the injection needle as a guide to placement within the target muscle. Injection without surgical exposure or electromyographic guidance should not be attempted. Physicians should be familiar with electromyographic technique.
To prepare the eye for BOTOX injection, it is recommended that several drops of a local anesthetic and an ocular decongestant be given several minutes prior to injection.
The volume of BOTOX injected for treatment of strabismus should be between 0.05-0.15 mL per muscle.
The initial listed doses of the reconstituted BOTOX [see Dosage and Administration (2.2)] typically create paralysis of the injected muscles beginning one to two days after injection and increasing in intensity during the first week. The paralysis lasts for 2-6 weeks and gradually resolves over a similar time period. Overcorrections lasting over six months have been rare. About one half of patients will require subsequent doses because of inadequate paralytic response of the muscle to the initial dose, or because of mechanical factors such as large deviations or restrictions, or because of the lack of binocular motor fusion to stabilize the alignment.
Initial Doses in Units
Use the lower listed doses for treatment of small deviations. Use the larger doses only for large deviations.
- For vertical muscles, and for horizontal strabismus of less than 20 prism diopters: 1.25 Units-2.5 Units in any one muscle.
- For horizontal strabismus of 20 prism diopters to 50 prism diopters: 2.5 Units-5 Units in any one muscle.
- For persistent VI nerve palsy of one month or longer duration: 1.25 Units-2.5 Units in the medial rectus muscle.
Subsequent Doses for Residual or Recurrent Strabismus
- It is recommended that patients be re-examined 7-14 days after each injection to assess the effect of that dose.
- Patients experiencing adequate paralysis of the target muscle that require subsequent injections should receive a dose comparable to the initial dose.
- Subsequent doses for patients experiencing incomplete paralysis of the target muscle may be increased up to two-fold compared to the previously administered dose.
- Subsequent injections should not be administered until the effects of the previous dose have dissipated as evidenced by substantial function in the injected and adjacent muscles.
- The maximum recommended dose as a single injection for any one muscle is 25 Units.
The recommended dilution to achieve 1.25 Units is 100 Units/8 mL; for 2.5 Units it is 100 Units/4 mL (see Table 1).