Drug Detail:Cimerli (Ranibizumab-eqrn (ophthalmic))
Generic Name: RANIBIZUMAB 0.3mg in 0.05mL
Dosage Form: injection, solution
Drug Class: Anti-angiogenic ophthalmic agents
Neovascular (Wet) Age-Related Macular Degeneration (AMD)
CIMERLI 0.5 mg (0.05 mL of 10 mg/mL solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
Although not as effective, patients may be treated with 3 monthly doses followed by less frequent dosing with regular assessment. In the 9 months after three initial monthly doses, less frequent dosing with 4-5 doses on average is expected to maintain visual acuity while monthly dosing may be expected to result in an additional average 1-2 letter gain. Patients should be assessed regularly [see Clinical Studies (14.1)] .
Although not as effective, patients may also be treated with one dose every 3 months after 4 monthly doses. Compared with continued monthly dosing, dosing every 3 months over the next 9 months will lead to an approximate 5-letter (1-line) loss of visual acuity benefit, on average. Patients should be assessed regularly [see Clinical Studies (14.1)] .
Macular Edema Following Retinal Vein Occlusion (RVO)
CIMERLI 0.5 mg (0.05 mL of 10 mg/mL solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
In Studies RVO-1 and RVO-2, patients received monthly injections of ranibizumab for 6 months. In spite of being guided by optical coherence tomography and visual acuity re-treatment criteria, patients who were then not treated at Month 6 experienced on average, a loss of visual acuity at Month 7, whereas patients who were treated at Month 6 did not. Patients should be treated monthly [see Clinical Studies (14.2)] .
Diabetic Macular Edema (DME) and Diabetic Retinopathy (DR)
CIMERLI 0.3 mg (0.05 mL of 6 mg/mL solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
Myopic Choroidal Neovascularization (mCNV)
CIMERLI 0.5 mg (0.05 mL of 10 mg/mL solution) is recommended to be initially administered by intravitreal injection once a month (approximately 28 days) for up to 3 months. Patients may be retreated if needed [(see Clinical Studies 14.5)] .
Preparation for Administration
Vial:
Using aseptic technique, all of the CIMERLI vial contents are withdrawn through a 5-micron (19-gauge × 1-1/2 inch), sterile filter needle attached to a 1 mL syringe (not included). The filter needle should be discarded after withdrawal of the vial contents and should not be used for intravitreal injection. The filter needle should be replaced with a sterile 30-gauge × 1/2 inch needle for the intravitreal injection.
Use aseptic technique to carry out the following preparation steps:
- Prepare for intravitreal injection with the following medical devices for use in a single eye (not included):
- a 5-micron sterile filter needle (19-gauge × 1-1/2 inch)
- a 1 mL sterile Luer lock syringe (with marking to measure 0.05 mL)
- a sterile injection needle (30-gauge × 1/2-inch)
- Before withdrawal, disinfect the outer part of the rubber stopper of the vial.
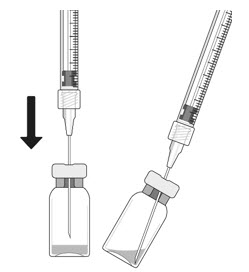
- Place a 5-micron filter needle (19-gauge × 1-1/2 inch) onto a 1 mL Luer lock syringe using aseptic technique.
- Push the filter needle into the center of the vial stopper until the needle touches the bottom edge of the vial.
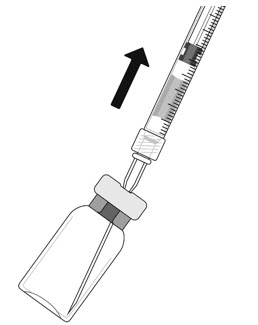
- Withdraw all the liquid from the vial, keeping the vial in an upright position, slightly inclined to ease complete withdrawal.
- Ensure that the plunger rod is drawn sufficiently back when emptying the vial in order to completely empty the filter needle.
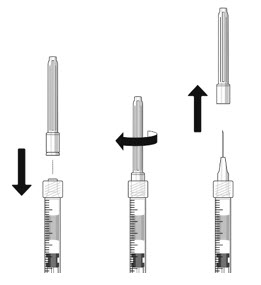
- The filter needle should be discarded after withdrawal of the vial contents and must not be used for the intravitreal injection.
- Attach a 30-gauge × 1/2-inch sterile injection needle firmly onto the syringe by screwing it tightly onto the Luer lock. Carefully remove the needle cap by pulling it straight off. Do not wipe the needle at any time.
- Hold the syringe with the needle pointing up. If there are any air bubbles, gently tap the syringe with your finger until the bubbles rise to the top.
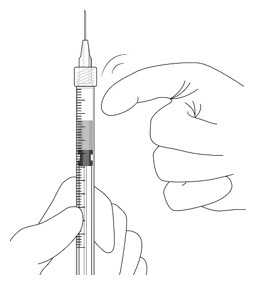
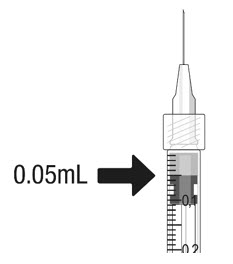
- Hold the syringe at eye level, and carefully push the plunger rod until the plunger tip is aligned with the line that marks 0.05 mL on the syringe.
Administration
The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
Prior to and 30 minutes following the intravitreal injection, patients should be monitored for elevation in intraocular pressure using tonometry. Monitoring may also consist of a check for perfusion of the optic nerve head immediately after the injection [see Warnings and Precautions (5.2)] . Patients should also be monitored for and instructed to report any symptoms suggestive of endophthalmitis without delay following the injection [see Warnings and Precautions (5.1)] .
Each vial should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter needle, and injection needles should be changed before CIMERLI is administered to the other eye.
No special dosage modification is required for any of the populations that have been studied (e.g., gender, elderly).









