Drug Detail:Eloxatin (Oxaliplatin [ ox-al-i-pla-tin ])
Generic Name: oxaliplatin 5mg in 1mL
Dosage Form: injection, solution, concentrate
Drug Class: Alkylating agents
ELOXATIN (oxaliplatin injection) should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate diagnostic and treatment facilities are readily available.
Dosage
Administer ELOXATIN in combination with 5-fluorouracil/leucovorin every 2 weeks. For advanced disease, treatment is recommended until disease progression or unacceptable toxicity. For adjuvant use, treatment is recommended for a total of 6 months (12 cycles):
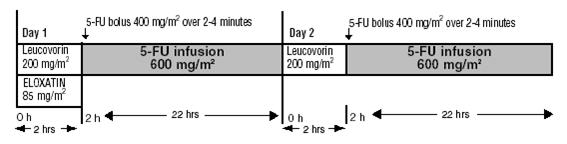
Day 1: ELOXATIN 85 mg/m2 intravenous infusion in 250–500 mL 5% Dextrose injection, USP and leucovorin 200 mg/m2 intravenous infusion in 5% Dextrose Injection, USP both given over 120 minutes at the same time in separate bags using a Y-line, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2–4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion.
Day 2: Leucovorin 200 mg/m2 intravenous infusion over 120 minutes, followed by 5-fluorouracil 400 mg/m2 intravenous bolus given over 2–4 minutes, followed by 5-fluorouracil 600 mg/m2 intravenous infusion in 500 mL 5% Dextrose Injection, USP (recommended) as a 22-hour continuous infusion.
Figure 1
The administration of ELOXATIN does not require prehydration. Premedication with antiemetics, including 5-HT3 blockers with or without dexamethasone, is recommended.
For information on 5-fluorouracil and leucovorin, see the respective package inserts.
Dose Modification Recommendations
Prior to subsequent therapy cycles, patients should be evaluated for clinical toxicities and recommended laboratory tests [see Warnings and Precautions (5.9)]. Prolongation of infusion time for ELOXATIN from 2 hours to 6 hours may mitigate acute toxicities. The infusion times for 5-fluorouracil and leucovorin do not need to be changed.
Adjuvant Therapy in Patients with Stage III Colon Cancer
Neuropathy and other toxicities were graded using the NCI CTC scale version 1 [see Warnings and Precautions (5.2)].
For patients who experience persistent Grade 2 neurosensory events that do not resolve, a dose reduction of ELOXATIN to 75 mg/m2 should be considered. For patients with persistent Grade 3 neurosensory events, discontinuing therapy should be considered. The infusional 5-fluorouracil/leucovorin regimen need not be altered.
A dose reduction of ELOXATIN to 75 mg/m2 and infusional 5-fluorouracil to 300 mg/m2 bolus and 500 mg/m2 22 hour infusion is recommended for patients after recovery from grade 3/4 gastrointestinal (despite prophylactic treatment), or grade 4 neutropenia, or febrile neutropenia, or grade 3/4 thrombocytopenia. The next dose should be delayed until: neutrophils ≥1.5 × 109/L and platelets ≥75 × 109/L.
Dose Modifications in Therapy in Previously Untreated and Previously Treated Patients with Advanced Colorectal Cancer
Neuropathy was graded using a study-specific neurotoxicity scale [see Warnings and Precautions (5.2)]. Other toxicities were graded by the NCI CTC, Version 2.0.
For patients who experience persistent Grade 2 neurosensory events that do not resolve, a dose reduction of ELOXATIN to 65 mg/m2 should be considered. For patients with persistent Grade 3 neurosensory events, discontinuing therapy should be considered. The 5-fluorouracil/leucovorin regimen need not be altered.
A dose reduction of ELOXATIN to 65 mg/m2 and 5-fluorouracil by 20% (300 mg/m2 bolus and 500 mg/m2 22-hour infusion) is recommended for patients after recovery from grade 3/4 gastrointestinal (despite prophylactic treatment), or grade 4 neutropenia, or febrile neutropenia, or grade 3/4 thrombocytopenia. The next dose should be delayed until: neutrophils ≥1.5 × 109/L and platelets ≥75 × 109/L.
Dose Modifications in Therapy for Patients with Renal Impairment
In patients with normal renal function or mild to moderate renal impairment, the recommended dose of ELOXATIN is 85 mg/m2. In patients with severe renal impairment, the initial recommended ELOXATIN dose should be reduced to 65 mg/m2 [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Preparation of Infusion Solution
Do not freeze and protect from light the concentrated solution.
A final dilution must never be performed with a sodium chloride solution or other chloride-containing solutions.
The solution must be further diluted in an infusion solution of 250-500 mL of 5% Dextrose Injection, USP.
After dilution with 250-500 mL of 5% Dextrose Injection, USP, the shelf life is 6 hours at room temperature [20-25°C (68-77°F)] or up to 24 hours under refrigeration [2-8°C (36-46°F)].
After final dilution, protection from light is not required.
ELOXATIN is incompatible in solution with alkaline medications or media (such as basic solutions of 5-fluorouracil) and must not be mixed with these or administered simultaneously through the same infusion line. The infusion line should be flushed with 5% Dextrose Injection, USP prior to administration of any concomitant medication.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and discarded if present.
Needles or intravenous administration sets containing aluminum parts that may come in contact with ELOXATIN should not be used for the preparation or mixing of the drug. Aluminum has been reported to cause degradation of platinum compounds.




