Drug Detail:Fasenra (Benralizumab [ ben-ra-liz-ue-mab ])
Generic Name: BENRALIZUMAB 30mg in 1mL
Dosage Form: injection, solution
Drug Class: Interleukin inhibitors
Recommended Dose
FASENRA is for subcutaneous use only.
The recommended dose of FASENRA is 30 mg administered once every 4 weeks for the first 3 doses, and then once every 8 weeks thereafter by subcutaneous injection into the upper arm, thigh, or abdomen.
General Administration Instructions
FASENRA is intended for use under the guidance of a healthcare provider. In line with clinical practice, monitoring of patients after administration of biologic agents is recommended [see Warnings and Precautions (5.1)].
Administer FASENRA into the thigh or abdomen. The upper arm can also be used if a healthcare provider or caregiver administers the injection. Prior to administration, warm FASENRA by leaving carton at room temperature for about 30 minutes. Visually inspect FASENRA for particulate matter and discoloration prior to administration. FASENRA is clear to opalescent, colorless to slightly yellow, and may contain a few translucent or white to off-white particles. Do not use FASENRA if the liquid is cloudy, discolored, or if it contains large particles or foreign particulate matter.
Prefilled Syringe
The prefilled syringe is for administration by a healthcare provider.
Autoinjector (FASENRA PEN™)
FASENRA PEN is intended for administration by patients/caregivers. Patients/caregivers may inject after proper training in subcutaneous injection technique, and after the healthcare provider determines it is appropriate.
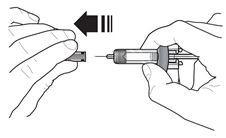
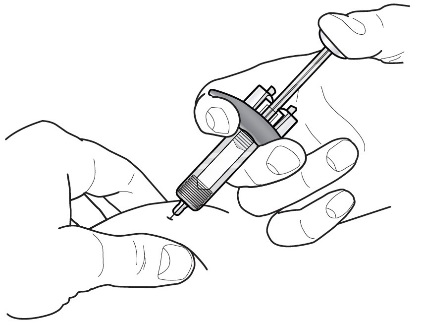
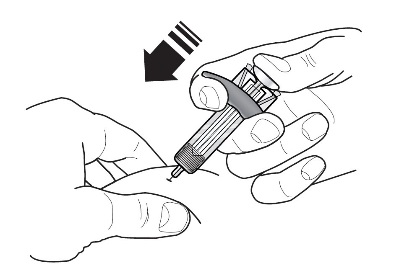
Instructions for Administration of FASENRA Prefilled Syringe (Healthcare Providers)
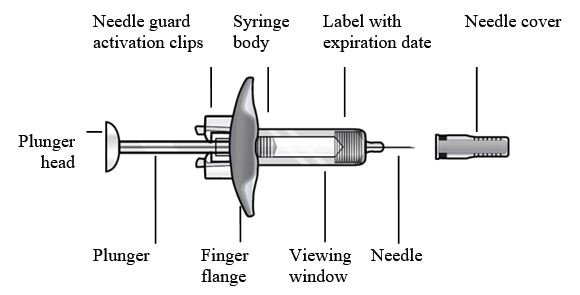
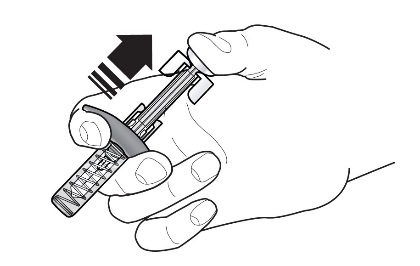
Refer to Figure 1 to identify the prefilled syringe components for use in the administration steps.
Figure 1
Do not touch the needle guard activation clips to prevent premature activation of the needle safety guard.
Instructions for Administration of FASENRA PEN
Refer to the FASENRA PEN ‘Instructions for Use’ for more detailed instructions on the preparation and administration of FASENRA PEN [See Instructions for Use]. A patient may self-inject or the patient caregiver may administer FASENRA PEN subcutaneously after the healthcare provider determines it is appropriate.