Drug Detail:Firmagon (Degarelix [ deg-a-rel-ix ])
Generic Name: DEGARELIX 40mg in 1mL;
Dosage Form: powder, metered, for subcutaneous injection
Drug Class: Gonadotropin-releasing hormone antagonists Hormones / antineoplastics
Dosing information
FIRMAGON is administered as a subcutaneous injection in the abdominal region only at the dosages in Table 1 below.
| Starting Dosage | Maintenance Dosage – Administered once every 28 days |
|---|---|
|
|
Reconstitution and Administration Instructions
FIRMAGON is to be administered by a healthcare professional only.
Before administering FIRMAGON read the Instructions for reconstitution and administration carefully.
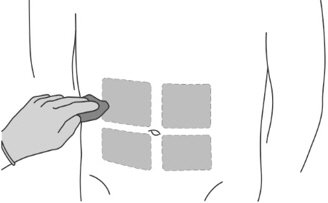
As with other drugs administered by subcutaneous injection, the injection site should vary periodically. Injections should be given only in areas of the abdomen that will not be exposed to pressure, e.g., not close to waistband or belt nor close to the ribs.
FIRMAGON is supplied as a powder to be reconstituted with Sterile Water for Injection, USP.
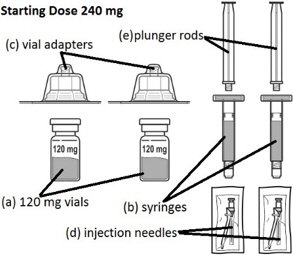
- Starting dose (240 mg): Two single-dose vials each delivering 120 mg of degarelix in a white to off-white lyophilized powder for reconstitution supplied with diluent in two prefilled syringes. Each vial is to be reconstituted with a prefilled syringe containing 3 mL of Sterile Water for Injection. 3 mL is withdrawn to deliver 120 mg degarelix at a concentration of 40 mg/mL.
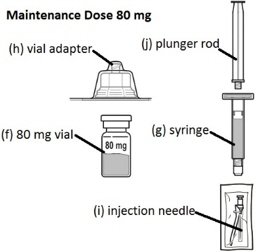
- Maintenance dose (80 mg): One single-dose vial delivering 80 mg of degarelix in a white to off-white lyophilized powder for reconstitution supplied with diluent in one prefilled syringe. Each vial is to be reconstituted with a prefilled syringe containing 4.2 mL of Sterile Water for Injection. 4 mL is withdrawn to deliver 80 mg degarelix at a concentration of 20 mg/mL.
Follow the instructions for reconstitution closely and read the complete instructions before performing the subcutaneous injection.
Reconstituted drug must be administered within one hour after addition of Sterile Water for Injection, USP.
Do not shake the vials.
Follow aseptic technique.
|
|
FIRMAGON 240 mg Starting Dose Kit contains:
|
|
|
FIRMAGON 80 mg Maintenance Dose Kit contains:
|
|
|
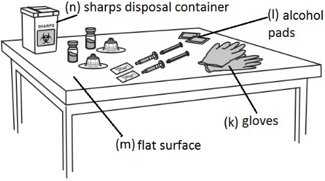
In addition the healthcare professional will need:
|
The drug product must be prepared using the following instructions:
NOTE: The mixing process must be repeated for the two injections of the Starting Dose prior to injecting the product into the patient's abdomen.
|
|
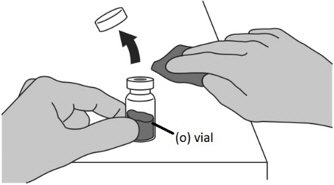
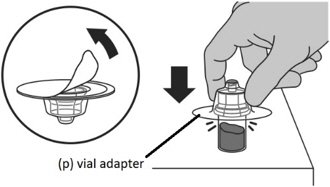
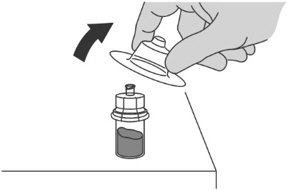
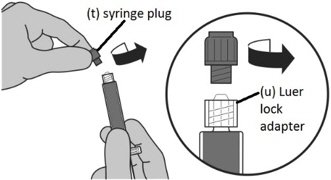
Step 1: Attaching the vial adaptor to the vial
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
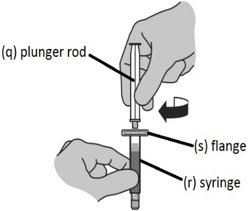
Step 2: Assembling the syringe
NOTE: You will only feel light resistance screwing the plunger rod in position. |
|
|
|
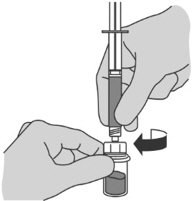
Step 3: Transferring Sterile Water for Injection, USP from the syringe to the vial
|
|
|
|
|
|
|
|
|
|
|
|
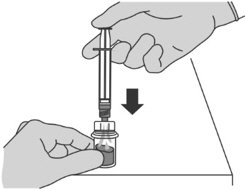
Step 4: Preparing the reconstituted injection
Reconstitution time can take up to 15 min but usually takes a few minutes. |
|
|
|
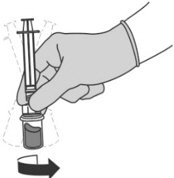
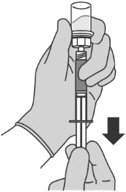
Step 5: Transferring the liquid to the syringe
|
|
|
|
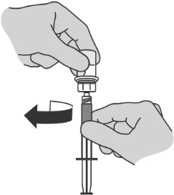
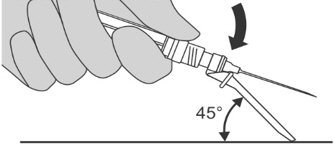
Step 6: Preparing the syringe for injection
|
|
|
|
|
|
|
|
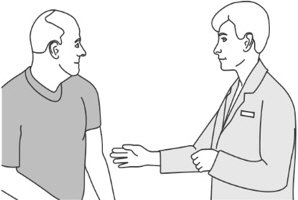
Step 7: Preparing the patient
|
|
|
||
|
||
|
|
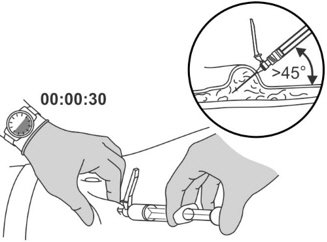
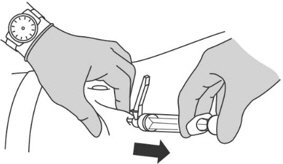
Step 8: Performing the injection
|
|
|
|
|
|
|
|
|
|
|
|
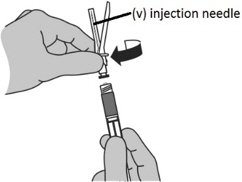
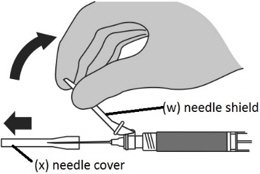
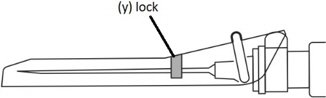
Step 9: Locking the needle into the shield
|
|
|
|
|
|
|
|
Step 10: Advising the patient
|
|
Disposing used needles and syringes
- Put used alcohol swabs, needles and syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away loose needles and syringes in the trash.
- For more information about safe sharps disposal, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.