Drug Detail:Haegarda (C1 esterase inhibitor subcutaneous (human) [ c1 es-ter-ase-in-hib-it-or ])
Generic Name: HUMAN C1-ESTERASE INHIBITOR 2000[iU] in 4mL;
Dosage Form: lyophilized powder in a single-use vial
Drug Class: Hereditary angioedema agents
After reconstitution, for subcutaneous use only.
HAEGARDA is intended for self (or caregiver)-administration after reconstitution at a dose of 60 International Units (IU) per kg body weight by subcutaneous (S.C.) injection twice weekly (every 3 or 4 days). The patient or caregiver should be trained on how to administer HAEGARDA.
HAEGARDA is provided as a freeze-dried powder for reconstitution with Sterile Water for Injection, USP.
Preparation and Handling
- Check the expiration date on the product vial label. Do not use beyond the expiration date.
- Work on a clean surface and wash hands before performing the following procedures.
- Prepare and administer using aseptic techniques [see Dosage and Administration (2.2)].
- Use a silicone-free syringe for reconstitution and administration.
- Each vial of HAEGARDA is for single-dose only. Promptly use the reconstituted solution. The solution must be used within 8 hours. Discard partially used vials. HAEGARDA contains no preservative.
- Do not freeze the reconstituted solution.
Reconstitution and Administration
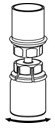
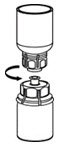
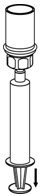
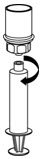
Use either the Mix2Vial® transfer set provided with HAEGARDA or a commercially available double-ended needle and vented filter spike [see How Supplied/Storage and Handling (16)].
Reconstitution
The procedures below are provided as general guidelines for the reconstitution and administration of HAEGARDA.
HAEGARDA Reconstitution Instructions
|
|
|
|
|
| Figure 1 |
|
| Figure 2 |
|
| Figure 3 |
|
| Figure 4 |
|
| Figure 5 |
|
| Figure 6 |
|
|
| Figure 7 |
|
| Figure 8 |
|
|
Administration
For subcutaneous injection only.
- Train the patient or caregiver on how to self-administer HAEGARDA.
- Do not mix HAEGARDA with other medicinal products.
- Visually inspect the final solution for particles and discoloration prior to administration, and whenever solution and container permit. Do not use if particles or discoloration is observed.
- Attach the syringe containing the reconstituted HAEGARDA solution to a hypodermic needle or subcutaneous infusion set and administer by subcutaneous injection. Adapt the rate of administration to the comfort level of the patient.
- Inject in the abdominal area or other subcutaneous injection sites. Rotate injection sites so that the same site is not used repeatedly.
- Administer HAEGARDA at room temperature and within 8 hours after reconstitution. Following administration, discard any unused solution and all administration equipment in an appropriate manner as per local requirements.