Drug Detail:Idelvion (Coagulation factor ix [ koe-ag-yoo-lay-shun-fak-tor-nine ])
Generic Name: ALBUTREPENONACOG ALFA 250[iU] in 2.5mL;
Dosage Form: injection
Drug Class: Miscellaneous coagulation modifiers
For intravenous use after reconstitution only.
Dosage
- Each single-dose vial of IDELVION contains the recombinant Factor IX potency in international units (IU) that is stated on the carton and vial label.
- Dosage and duration of treatment with IDELVION depends on the severity of Factor IX deficiency, the location and extent of bleeding, and the patient's clinical condition, age and recovery of Factor IX.
- The calculation of the required dose of IDELVION is based on the empirical finding that one IU of IDELVION per kg body weight is expected to increase the circulating level of Factor IX by 1.3 IU/dL in patients ≥12 years of age and by 1 IU/dL in patients <12 years of age. The required dose of IDELVION for treatment of bleeding episodes is determined using the following formula:
Required Units (IU) = Body Weight (kg) × Desired Factor IX rise (% of normal or IU/dL) × (reciprocal of recovery (IU/kg per IU/dL)) OR Increase in Factor IX IU/dL (or % of normal) = Dose (IU) × Recovery (IU/dL per IU/kg)/body weight (kg) - Adjust the dose based on the individual patient's clinical condition and response.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing IDELVION for the on-demand treatment and control of bleeding episodes is provided in Table 1. Dosing should aim at maintaining a plasma Factor IX activity level at or above the plasma levels (in % of normal or IU/dL) outlined in Table 1.
| Type of Bleeding Episode | Circulating Factor IX Activity Required [% or (IU/dL)] |
Frequency of Dosing (hours) | Duration of Therapy (days)* |
|---|---|---|---|
|
|||
| Minor or Moderate Uncomplicated hemarthrosis, muscle bleeding (except iliopsoas) or oral bleeding |
30-60 | 48-72 | At least 1 day, until bleeding stops and healing is achieved. Single dose should be sufficient for majority of bleeds. |
| Major Life or limb threatening hemorrhage, deep muscle bleeding, including iliopsoas, intracranial, retropharyngeal |
60-100 | 48-72 | 7-14 days, until bleeding stops and healing is achieved. Maintenance dose weekly. |
Perioperative Management of Bleeding
A guide for dosing IDELVION for perioperative management of bleeding is provided in Table 2.
| Type of Surgery | Circulating Factor IX Activity Required [% or (IU/dL)] |
Frequency of Dosing (hours) | Duration of Therapy (days)* |
|---|---|---|---|
|
|||
| Minor (including uncomplicated tooth extraction) |
50-80 | 48-72 | At least 1 day, or until healing is achieved. Single dose should be sufficient for a majority of minor surgeries. |
| Major (including intracranial, pharyngeal, retropharyngeal, retroperitoneal) |
60-100 (initial level) |
48-72 | 7-14 days, or until bleeding stops and healing is achieved. Repeat dose every 48-72 hours for the first week or until healing is achieved. Maintenance dose 1-2 times per week. |
Routine Prophylaxis
For patients ≥12 years of age, the recommended dose is 25-40 IU IDELVION per kg body weight every 7 days. Patients who are well-controlled on a 7-day regimen may be switched to a 14-day interval at 50-75 IU IDELVION per kg body weight [see Clinical Studies (14)].
For patients <12 years of age, the recommended dose is 40-55 IU IDELVION per kg body weight every 7 days.
- A regimen may be further individually adjusted to less or more frequent dosing in adults.
Preparation and Reconstitution
The procedures below are provided as general guidelines for the preparation and reconstitution of IDELVION.
- Always work on a clean surface and wash your hands before performing the following procedures.
- Use aseptic technique during the reconstitution procedure.
- Reconstitute IDELVION using the diluent (Sterile Water for Injection) and transfer device (Mix2Vial) provided in the kit.
- To administer, you will also need a syringe and suitable needle (not provided).
- Ensure the vials of IDELVION and the Sterile Water for Injection are at room temperature before mixing.
- The reconstitution is performed as described below.
IDELVION Reconstitution Instructions
- Place the IDELVION vial, diluent vial, and Mix2Vial® transfer set on a flat surface.
- Remove flip caps from the IDELVION and Sterile Water for Injection (diluent) vials.
- Wipe the stoppers with the sterile alcohol swab provided and allow to dry prior to opening the Mix2Vial transfer set package.
- Open the Mix2Vial transfer set package by peeling away the lid (Fig. 1). Do not remove the device from the package.
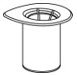
Fig. 1 - Place the diluent vial on a flat surface and hold the vial tightly. Grip the Mix2Vial transfer set together with the clear package and push the plastic spike at the blue end of the Mix2Vial transfer set firmly through the center of the stopper of the diluent vial (Fig. 2).
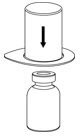
Fig. 2 - Carefully remove the clear package from the Mix2Vial transfer set. Do not remove the Mix2Vial transfer set or touch the exposed end of the device (Fig. 3).
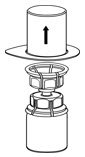
Fig. 3 - With the IDELVION vial placed firmly on a flat surface, invert the diluent vial with the Mix2Vial transfer set attached and push the plastic spike of the transparent adapter firmly through the center of the stopper of the IDELVION vial (Fig. 4). The diluent will automatically transfer into the IDELVION vial.
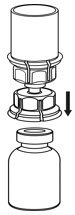
Fig. 4 - With the diluent and IDELVION vial still attached to the Mix2Vial transfer set, gently swirl the IDELVION vial to ensure that the powder is fully dissolved (Fig. 5). Do not shake the vial.
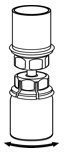
Fig. 5 - With one hand, grasp the IDELVION side of the Mix2Vial transfer set and with the other hand grasp the blue diluent-side of the Mix2Vial transfer set, and unscrew the set into two pieces (Fig. 6).
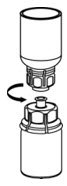
Fig. 6 - Draw air into an empty, sterile syringe. While the IDELVION vial is upright, screw the syringe to the Mix2Vial transfer set. Inject air into the IDELVION vial.
- While keeping the syringe plunger pressed, invert the system upside down and draw the concentrate into the syringe by pulling the plunger back slowly (Fig. 7).
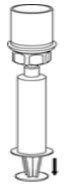
Fig. 7 - Disconnect the filled syringe by unscrewing it from the Mix2Vial transfer set (Fig. 8). The reconstituted solution should be a clear or yellow to colorless solution. Do not use if particulate matter or discoloration is observed.
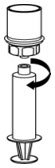
Fig. 8 - Use immediately or within 4 hours of reconstitution. Store reconstituted solution at room temperature. Do not refrigerate.
- If the dose requires more than one vial, use a separate, unused Mix2Vial transfer set and Sterile Water for Injection (diluent) vial for each product vial. Repeat steps 10-12 to pool the contents of the vials into one syringe.
Administration
For intravenous injection only.
- Do not mix or administer IDELVION in the same tubing or container with other medicinal products.
- Visually inspect the final solution for particulate matter and discoloration prior to administration, and whenever solution and container permit. Do not use if particulate matter or discoloration is observed.
- Attach the syringe containing the reconstituted IDELVION solution to a sterile infusion set and administer by intravenous injection. Adapt the infusion rate to the comfort level of each patient, not exceeding 10 mL per minute.
- Administer IDELVION at room temperature and within 4 hours of reconstitution. Discard any unused product.












