Drug Detail:Infed (Iron dextran [ eye-urn-dex-tran ])
Generic Name: IRON DEXTRAN 50mg in 1mL
Dosage Form: injection
Drug Class: Iron products
2.1 Important Pre-Treatment Information
Discontinue administration of any iron-containing products prior to administration of INFeD.
Assess baseline hematologic (hemoglobin and hematocrit) and iron storage parameters (serum iron, total iron binding capacity, and percent saturation of transferrin) to monitor response to therapy.
Administer a test dose of INFeD prior to administration of therapeutic dose [see Dosage and Administration (2.4)].
2.2 Recommended Dosage for Iron Deficiency Anemia
Calculate the INFeD dose based upon Table 1 and formulas below. Continue INFeD until hemoglobin is within the normal range and iron stores are replete.
Administer daily doses of no more than 2 mL of INFeD until the total required dose is administered. Monitor response to therapy by evaluating hematologic parameters (hemoglobin and hematocrit) and iron storage parameters (serum iron, total iron binding capacity, and percent saturation of transferrin). Iron storage parameters may improve prior to hematologic parameters. Serum ferritin may not be an accurate measure of body iron stores in patients on chronic dialysis.
Table 1: Total INFeD Requirement for Hemoglobin Restoration and Iron Stores Replacement in Patients with Iron Deficiency Anemia*
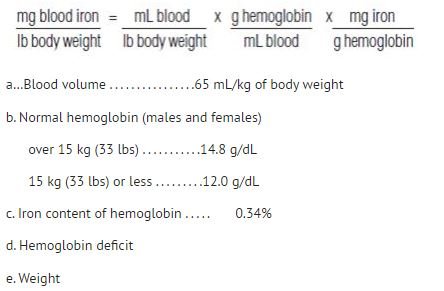
Alternatively, the total dose may be calculated using the formulas below:
Adults and Children over 15 kg (33 lbs)
Dose (mL) = 0.0442 (Desired Hb - Observed Hb) x LBW + (0.26 x LBW)
Based on:
• Desired Hb = the target hemoglobin in g/dL [Normal hemoglobin (males and females) for body weight over 15 kg (33 lbs) is 14.8 g/dL.]
• Observed Hb = the patient’s current hemoglobin in g/dL
• LBW = Lean body weight in kg [A patient’s lean body weight (or actual body weight if less than lean body weight) should be utilized when determining dosage.]
• For males: LBW = 50 kg + 2.3 kg for each inch of patient’s height over 5 feet
• For females: LBW = 45.5 kg + 2.3 kg for each inch of patient’s height over 5 feet
• To calculate a patient's weight in kg when lbs are known:
Weight in Kg
Children 5 to 15 kg (11 to 33 lbs)
Otherwise, the total dose may be calculated using the formula below:
Dose (mL) = 0.0442 (Desired Hb - Observed Hb) x W + (0.26 x W)
Based on:
• Desired Hb = the target hemoglobin in g/dL [Normal hemoglobin for children with body weight of 15 kg (33 lbs) or less is 12 g/dL.]
• W = body weight in kg
• To calculate a patient's weight in kg when lbs are known:
Weight in Kg
2.3 Recommended Dosage of Iron Replacement for Blood Loss
Calculate the INFeD dose based upon the formula below which is based upon the approximate amount of blood loss and pretreatment hematocrit.
The formula is based on the approximation that 1 mL of normocytic, normochromic red cells contains 1 mg of elemental iron.
INFeD Dose (in mL) = [Blood loss (in mL) x hematocrit] ÷ 50 mg/mL
Example: Blood loss of 500 mL with 20% hematocrit
Replacement Iron = 500 x 0.20 = 100 mg
INFeD dose volume = INFeD dose volume
2.4 Administration
The total volume of INFeD required for the treatment of iron deficiency anemia is determined from Table 1 or the appropriate formula listed [see Dosage and Administration (2.2)].
The total volume of INFeD required for the treatment of iron replacement for blood loss is determined from an appropriate formula listed [see Dosage and Administration (2.3)].
NOTE: Do not mix INFeD with other medications or add to parenteral nutrition solutions for intravenous infusion.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever the solution and container permit.
Discard unused portion.
Intravenous Injection
Test Dose
Prior to the first intravenous INFeD therapeutic dose, administer an intravenous test dose of 0.5 mL [see BOXED WARNING and Warnings and Precautions (5.1)]. Administer the test dose at a gradual rate over at least 30 seconds. Delay administration of the initial therapeutic INFeD dose until 1 hour or more after the test dose. If a hypersensitivity reaction occurs with the test dose, manage medically and do not administer further doses of INFeD.
INFeD is given undiluted at a slow gradual rate not to exceed 50 mg (1 mL) per minute.
The maximum daily dose of INFeD should not exceed 2 mL.
Intramuscular Injection
Test Dose
Prior to the first intramuscular INFeD therapeutic dose, administer an intramuscular test dose of 0.5 mL [see BOXED WARNING and Warnings and Precautions (5.1)]. Administer the test dose at a gradual rate over at least 30 seconds into the buttock. Delay administration of the initial therapeutic INFeD dose until 1 hour or more after the test dose. If a hypersensitivity reaction occurs with the test dose, manage medically and do not administer further doses of INFeD.
If no adverse reactions are observed, INFeD can be given according to the following schedule until the calculated total required dose has been reached. Each day’s dose should not exceed 0.5 mL (25 mg of iron) for infants with body weight under 5 kg (11 lbs); 1 mL (50 mg of iron) for children with body weight under 10 kg (22 lbs); and 2 mL (100 mg of iron) for other patients.
The maximum daily dose of INFeD should not exceed 2 mL.
INFeD should be injected only into the muscle mass of the upper outer quadrant of the buttock - never into the arm or other exposed areas - and should be injected deeply, with a 2-inch or 3-inch 19 or 20 gauge needle. If the patient is standing, he/she should be bearing his/her weight on the leg opposite the injection site, or if in bed, he/she should be in the lateral position with injection site uppermost. To avoid injection or leakage into the subcutaneous tissue, a Z-track technique (displacement of the skin laterally prior to injection) is recommended.




