Drug Detail:Infumorph (injection) (Morphine (injection) [ mor-feen ])
Generic Name: MORPHINE SULFATE 10mg in 1mL
Dosage Form: injection, solution
Drug Class: Opioids (narcotic analgesics)
Important Dosage and Administration Instructions
INFUMORPH should be administered by or under the direction of a physician experienced in the techniques of epidural or intrathecal administration and familiar with the patient management problems associated with epidural or intrathecal drug administration.
- Because of the risk of delayed respiratory depression, patients should be observed in a fully equipped and staffed environment for at least 24 hours after each test dose and, as indicated, for the first several days after surgery.
- Because epidural administration has been associated with less potential for immediate or late adverse effects than intrathecal administration, the epidural route should be used whenever possible.
- For safety reasons, it is recommended that administration of INFUMORPH 200 and 500 (10 and 25 mg/mL, respectively) by the intrathecal route be limited to the lumbar area.
- INFUMORPH 200 and 500 (10 and 25 mg/mL, respectively) should not be used for single-dose neuraxial injection because lower doses can be more reliably administered with the standard preparation of DURAMORPH (0.5 and 1 mg/mL).
Candidates for neuraxial administration of INFUMORPH in a continuous microinfusion device should be hospitalized to provide for adequate patient monitoring during assessment of response to single doses of intrathecal or epidural morphine. Hospitalization should be maintained for several days after surgery involving the infusion device for additional monitoring and adjustment of daily dosage. The facility must be equipped with resuscitative equipment, oxygen, naloxone injection and other resuscitative drugs.
A period of observation appropriate to the clinical situation should follow each refill or manipulation of the drug reservoir. Before discharge, the patient and attendant(s) should receive instruction in the proper home care of the device and insertion site and in the recognition and practical treatment of an overdose of neuraxial morphine.
Familiarization with the continuous microinfusion device is essential. The desired amount of morphine should be withdrawn from the ampul through a microfilter. To minimize risk from glass or other particles, the product must be filtered through a 5 µ (or smaller) microfilter before injecting into the microinfusion device. If dilution is required, 0.9% Sodium Chloride Injection is recommended.
Reservoir filling must be performed by fully trained and qualified personnel, following the directions provided by the device manufacturer. Care should be taken in selecting the proper refill frequency to prevent depletion of the reservoir, which would result in exacerbation of severe pain, onset of opioid withdrawal symptoms, and/or reflux of cerebrospinal fluid into some devices. Strict aseptic technique is required to avoid bacterial contamination and serious infection. Extreme care must be taken to ensure that the needle is properly inserted into the filling port of the device before attempting to refill the reservoir. Injecting the solution into the tissue around the device or (in the case of devices that have more than one port) attempting to inject the refill dose into the direct injection port will result in a large, clinically significant, overdosage to the patient.
Safety and Handling Instructions:
INFUMORPH is supplied in sealed ampuls. Accidental dermal exposure should be treated by the removal of any contaminated clothing and rinsing the affected area with water.
Inspect parenteral drug products for particulate matter before opening the amber ampul and again for color after removing contents from the ampul. Do not use if the solution in the unopened ampul contains a precipitate which does not disappear upon shaking. After removal, do not use unless the solution is colorless or pale yellow.
INFUMORPH is intended for single-use only. Protect from light, discard any unused portion. Do not heat-sterilize.
Initial Dosage
The starting dose of INFUMORPH must be individualized, based upon in-hospital evaluation of the response to serial single-dose epidural or intrathecal bolus injections of regular DURAMORPH (morphine sulfate injection,) 0.5 mg/mL or 1 mg/mL, with close observation for analgesic efficacy and adverse effects prior to surgery involving the continuous microinfusion device.
- Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5.3)].
- Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.3)].
- Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with INFUMORPH and adjust the dosage accordingly [see Warnings and Precautions (5.2)].
Dosage for Epidural Administration
The recommended initial epidural dose in patients who are not tolerant to opioids ranges from 3.5 to 7.5 mg/day. The usual starting dose for continuous epidural infusion, based upon limited data in patients who have some degree of opioid tolerance, is 4.5 to 10 mg/day. The dose requirements may increase significantly during treatment, frequently to 20-30 mg/day. The upper daily limit for each patient must be individualized.
Dosage for Intrathecal Administration
The recommended initial lumbar intrathecal dose range in patients with no tolerance to opioids is 0.2 to 1 mg/day. The published range of doses for individuals who have some degree of opioid tolerance varies from 1 to 10 mg/day. The upper daily dosage limit for each patient must be individualized.
- Intrathecal dosage is usually 1/10 that of epidural dosage.
Titration and Maintenance of Therapy
Individually titrate INFUMORPH to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving INFUMORPH to assess the maintenance of pain control and the relative incidence of adverse reactions, as well as monitoring for the development of addiction, abuse, or misuse [see Warnings and Precautions (5.3)]. Frequent communication is important among the prescriber, other members of the healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration.
If the level of pain increases after dosage stabilization, attempt to identify the source of the increased pain before increasing the INFUMORPH dosage. Limited experience with continuous intrathecal infusion of morphine has shown that the daily doses have to be increased over time. Although the rate of increase, over time, in the dose required to sustain analgesia is highly variable, an estimate of the expected rate of increase is shown in the following Figure.
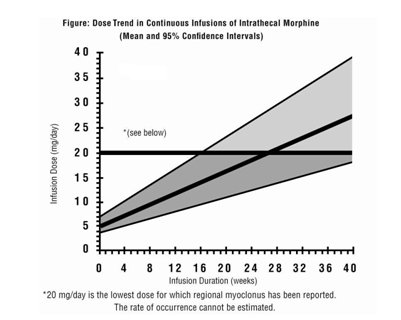
Doses above 20 mg/day should be employed with caution since they may be associated with a higher likelihood of serious side effects [see Warnings and Precautions (5.2, 5.7) and Adverse Reactions (6)].
If unacceptable opioid-related adverse reactions are observed, consider reducing the dosage. Adjust the dosage to obtain an appropriate balance between management of pain and opioid-related adverse reactions.
Discontinuation of INFUMORPH
When a patient who has been taking INFUMORPH regularly and may be physically dependent or no longer requires therapy with INFUMORPH, taper the dose gradually while monitoring carefully for signs and symptoms of withdrawal. If the patient develops these signs or symptoms, raise the dose to the previous level and taper more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both. Do not stop INFUMORPH abruptly in a physically-dependent patient [see Warnings and Precautions (5.15), Drug Abuse and Dependence (9.3)].




