Drug Detail:Jetrea (Ocriplasmin)
Generic Name: OCRIPLASMIN 1.25mg in 1mL
Dosage Form: injection, solution
Drug Class: Miscellaneous ophthalmic agents
General Dosing Information
For single-use ophthalmic intravitreal injection only. JETREA must only be administered by a qualified physician.
This formulation of JETREA does not require dilution.
Dosing
The recommended dose is 0.125 mg (0.1 mL of the solution) administered by intravitreal injection to the affected eye once as a single dose.
Preparation for Administration
To prepare JETREA for intravitreal injection, adhere to the following instructions:
-
Remove the vial (1.25 mg/mL corresponding to 0.375 mg ocriplasmin) from the freezer and allow to thaw at room temperature (within a few minutes). Use JETREA immediately after thawing. Unopened vials in the original carton protected from light can be stored up to 8 hours when stored below 77°F (25°C). Do not refreeze a vial once it has been thawed.
-
Once completely thawed, remove the protective polypropylene blue flip-off cap from the vial (see Figure 1) .
Figure 1:
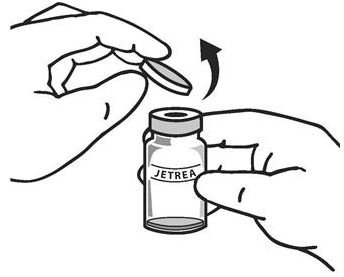
-
. The top of the vial should be disinfected with an alcohol wipe (see Figure 2) .
Figure 2:
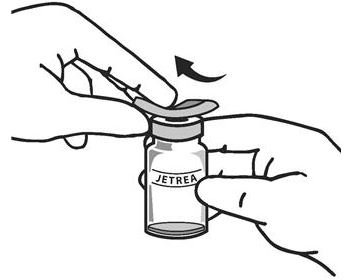
-
Visually inspect the vial for particulate matter. Only a clear, colorless solution without visible particles should be used.
-
Using aseptic technique, withdraw all of the solution using a sterile #19 gauge needle (slightly tilt the vial to ease withdrawal) and discard the needle after withdrawal of the vial contents (see Figure 3). Do not use this needle for the intravitreal injection.
Figure 3:
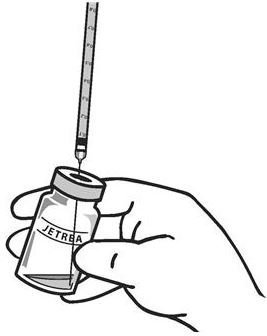
-
. Replace the needle with a sterile #30 gauge needle, carefully expel the air bubbles and excess drug from the syringe and adjust the dose to the 0.1 mL mark on the syringe (corresponding to 0.125 mg ocriplasmin) (see Figure 4).
Figure 4:
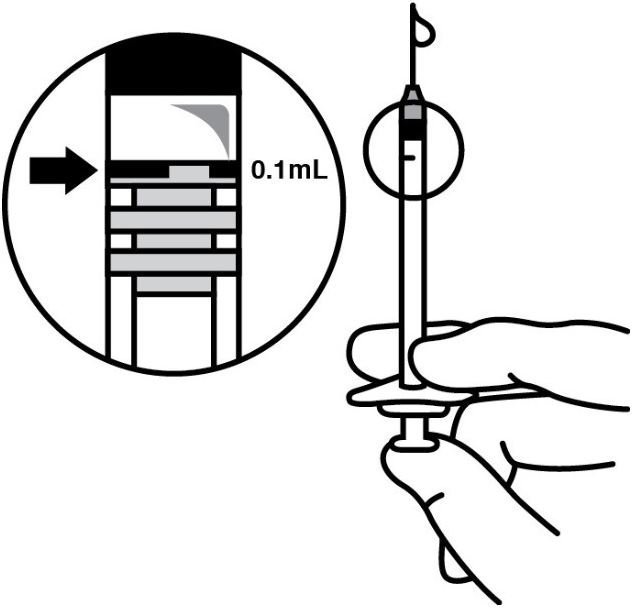
-
THE SOLUTION SHOULD BE USED IMMEDIATELY AS IT CONTAINS NO PRESERVATIVES.
-
Discard the vial and any unused portion of the solution after single use.
Administration and Monitoring
The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include the use of sterile gloves, a sterile drape and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad spectrum microbiocide should be administered according to standard medical practice.
The injection needle should be inserted 3.5 - 4.0 mm posterior to the limbus aiming towards the center of the vitreous cavity, avoiding the horizontal meridian. The injection volume of 0.1 mL is then delivered into the mid-vitreous.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure. Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, a sterile paracentesis needle should be available.
. Following intravitreal injection, patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment (e.g., eye pain, redness of the eye, photophobia, blurred or decreased vision) without delay [see Patient Counseling Information (17)] .
Each vial should only be used to provide a single injection for the treatment of a single eye. If the contralateral eye requires treatment, a new vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, and injection needles should be changed before JETREA is administered to the other eye, however, treatment with JETREA in the other eye is not recommended within 7 days of the initial injection in order to monitor the post-injection course including the potential for decreased vision in the injected eye.
Repeated administration of JETREA in the same eye is not recommended [see Nonclinical Toxicology (13.2)] .
After injection, any unused product must be discarded.
No special dosage modification is required for any of the populations that have been studied (e.g. gender, elderly).








