Drug Detail:Lucentis (Ranibizumab (ophthalmic) [ ra-nib-i-zue-mab-off-thal-mik ])
Generic Name: RANIBIZUMAB 10mg in 1mL
Dosage Form: injection, solution
Drug Class: Anti-angiogenic ophthalmic agents
Neovascular (Wet) Age-Related Macular Degeneration (AMD)
LUCENTIS 0.5 mg (0.05 mL of 10 mg/mL solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
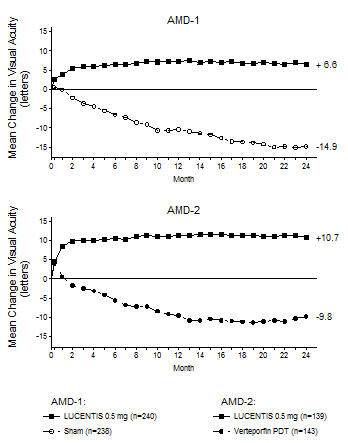
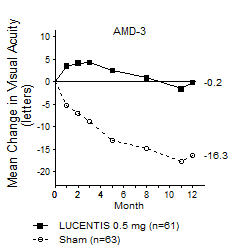
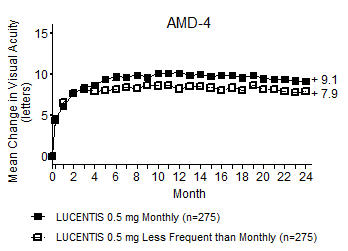
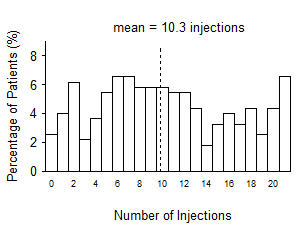
Although not as effective, patients may be treated with 3 monthly doses followed by less frequent dosing with regular assessment. In the 9 months after three initial monthly doses, less frequent dosing with 4-5 doses on average is expected to maintain visual acuity while monthly dosing may be expected to result in an additional average 1-2 letter gain. Patients should be assessed regularly [see Clinical Studies (14.1)].
Although not as effective, patients may also be treated with one dose every 3 months after 4 monthly doses. Compared with continued monthly dosing, dosing every 3 months over the next 9 months will lead to an approximate 5-letter (1-line) loss of visual acuity benefit, on average. Patients should be assessed regularly [see Clinical Studies (14.1)].
Macular Edema Following Retinal Vein Occlusion (RVO)
LUCENTIS 0.5 mg (0.05 mL of 10 mg/mL solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
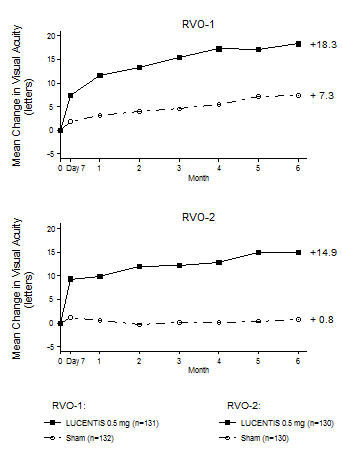
In Studies RVO-1 and RVO-2, patients received monthly injections of LUCENTIS for 6 months. In spite of being guided by optical coherence tomography and visual acuity re-treatment criteria, patients who were then not treated at Month 6 experienced on average, a loss of visual acuity at Month 7, whereas patients who were treated at Month 6 did not. Patients should be treated monthly [see Clinical Studies (14.2)].
Diabetic Macular Edema (DME) and Diabetic Retinopathy (DR)
LUCENTIS 0.3 mg (0.05 mL of 6 mg/mL solution) is recommended to be administered by intravitreal injection once a month (approximately 28 days).
Myopic Choroidal Neovascularization (mCNV)
LUCENTIS 0.5 mg (0.05 mL of 10 mg/mL LUCENTIS solution) is recommended to be initially administered by intravitreal injection once a month (approximately 28 days) for up to 3 months. Patients may be retreated if needed [(see Clinical Studies 14.5)].
Preparation for Administration
Prefilled Syringe:
The prefilled syringe is sterile and is for single use only. Do not use the product if the packaging is damaged or has been tampered with.
To prepare LUCENTIS for intravitreal administration, please adhere to these instructions for use. Read all the instructions carefully before using the prefilled syringe.
The opening of the sealed tray and all subsequent steps should be done under aseptic conditions.
For the intravitreal injection, a 30-gauge × ½ inch sterile injection needle should be used (not provided).
Note: the dose must be set to 0.05 mL.
| Device description | |
LUCENTIS prefilled syringes are available in 2 dose strengths:
|
|
 |
 |
|
|
 |
 |
| Check the labels on the LUCENTIS carton, syringe tray and prefilled syringe to make sure you have the correct dose strength.
|
|
| Step 1: Prepare | |
|
|
| Step 2: Inspect syringe | |
|
|
| Step 3: Remove syringe cap | |
|
|
| Step 4: Attach needle | |
Note: Do not wipe the needle at any time. |
|
| Step 5: Dislodge air bubbles | |
|
|
| Step 6: Expel air and adjust drug dose | |
|
|
| Step 7: Inject | |
|
|
Vial:
Using aseptic technique, all of the LUCENTIS vial contents are withdrawn through a 5-micron (19-gauge × 1-1/2 inch), sterile filter needle attached to a 1 mL syringe (not included). The filter needle should be discarded after withdrawal of the vial contents and should not be used for intravitreal injection. The filter needle should be replaced with a sterile 30-gauge × ½ inch needle for the intravitreal injection.
Use aseptic technique to carry out the following preparation steps:
- Prepare for intravitreal injection with the following medical devices for single use (not included):
- a 5-micron sterile filter needle (19-gauge × 1-1/2 inch)
- a 1 mL sterile Luer lock syringe (with marking to measure 0.05 mL)
- a sterile injection needle (30-gauge × 1/2-inch)
- Before withdrawal, disinfect the outer part of the rubber stopper of the vial.
- Place a 5-micron filter needle (19-gauge × 1-1/2 inch) onto a 1 mL Luer lock syringe using aseptic technique.
- Push the filter needle into the center of the vial stopper until the needle touches the bottom edge of the vial.
- Withdraw all the liquid from the vial, keeping the vial in an upright position, slightly inclined to ease complete withdrawal.
- Ensure that the plunger rod is drawn sufficiently back when emptying the vial in order to completely empty the filter needle.
- The filter needle should be discarded after withdrawal of the vial contents and must not be used for the intravitreal injection.
- Attach a 30-gauge × 1/2-inch sterile injection needle firmly onto the syringe by screwing it tightly onto the Luer lock. Carefully remove the needle cap by pulling it straight off. Do not wipe the needle at any time.
- Hold the syringe with the needle pointing up. If there are any air bubbles, gently tap the syringe with your finger until the bubbles rise to the top.
- Hold the syringe at eye level, and carefully push the plunger rod until the plunger tip is aligned with the line that marks 0.05 mL on the syringe.
Administration
The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include the use of sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
Prior to and 30 minutes following the intravitreal injection, patients should be monitored for elevation in intraocular pressure using tonometry. Monitoring may also consist of a check for perfusion of the optic nerve head immediately after the injection [see Warnings and Precautions (5.2)]. Patients should also be monitored for and instructed to report any symptoms suggestive of endophthalmitis without delay following the injection [see Warnings and Precautions (5.1)].
Each prefilled syringe or vial should only be used for the treatment of a single eye. If the contralateral eye requires treatment, a new prefilled syringe or vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, filter needle (vial only), and injection needles should be changed before LUCENTIS is administered to the other eye.
No special dosage modification is required for any of the populations that have been studied (e.g., gender, elderly).














