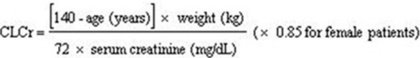Drug Detail:Neurontin (Gabapentin [ ga-ba-pen-tin ])
Generic Name: GABAPENTIN 100mg
Dosage Form: capsules, tablets, oral solution
Drug Class: Gamma-aminobutyric acid analogs
Dosage for Postherpetic Neuralgia
In adults with postherpetic neuralgia, NEURONTIN may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). The dose can subsequently be titrated up as needed for pain relief to a dose of 1800 mg/day (600 mg three times a day). In clinical studies, efficacy was demonstrated over a range of doses from 1800 mg/day to 3600 mg/day with comparable effects across the dose range; however, in these clinical studies, the additional benefit of using doses greater than 1800 mg/day was not demonstrated.
Dosage for Epilepsy with Partial Onset Seizures
Patients 12 years of age and above
The starting dose is 300 mg three times a day. The recommended maintenance dose of NEURONTIN is 300 mg to 600 mg three times a day. Dosages up to 2400 mg/day have been well tolerated in long-term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours.
Pediatric Patients Age 3 to 11 years
The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in three divided doses, and the recommended maintenance dose reached by upward titration over a period of approximately 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours.
Dosage Adjustment in Patients with Renal Impairment
Dosage adjustment in patients 12 years of age and older with renal impairment or undergoing hemodialysis is recommended, as follows (see dosing recommendations above for effective doses in each indication):
| TID = Three times a day; BID = Two times a day; QD = Single daily dose | ||||||
|
||||||
|
Renal Function Creatinine Clearance |
Total Daily Dose Range |
Dose Regimen |
||||
|
≥ 60 |
900 to 3600 |
300 TID |
400 TID |
600 TID |
800 TID |
1200 TID |
|
>30 to 59 |
400 to 1400 |
200 BID |
300 BID |
400 BID |
500 BID |
700 BID |
|
>15 to 29 |
200 to 700 |
200 QD |
300 QD |
400 QD |
500 QD |
700 QD |
|
15* |
100 to 300 |
100 QD |
125 QD |
150 QD |
200 QD |
300 QD |
|
Post-Hemodialysis Supplemental Dose (mg)† |
||||||
|
Hemodialysis |
125† |
150† |
200† |
250† |
350† |
|
Creatinine clearance (CLCr) is difficult to measure in outpatients. In patients with stable renal function, creatinine clearance can be reasonably well estimated using the equation of Cockcroft and Gault:
The use of NEURONTIN in patients less than 12 years of age with compromised renal function has not been studied.
Dosage in Elderly
Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and dose should be adjusted based on creatinine clearance values in these patients.
Administration Information
Administer NEURONTIN orally with or without food.
NEURONTIN capsules should be swallowed whole with water.
Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded.
If the NEURONTIN dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber).