Drug Detail:Ryplazim ( plasminogen, human-tvmh)
Generic Name: Plasminogen 68.8mg
Dosage Form: injection, powder, lyophilized, for solution
Drug Class: Miscellaneous uncategorized agents
For intravenous use after reconstitution only.
Dosage
Dose Determination
The recommended dosage of RYPLAZIM is 6.6 mg/kg body weight administered intravenously every 2 to 4 days (Q2D to Q4D).
Calculate the total infusion volume of RYPLAZIM using Formula (1), which is based on a final plasminogen concentration of 5.5 mg/mL. Administer the exact infusion volume determined using Formula (1) to the patient.
| Formula (1): | Infusion volume (mL) = body weight (kg) × 1.2 |
May require more than one reconstituted vial of RYPLAZIM to obtain the infusion volume calculated using Formula (1). Round up the estimated number of vials using Formula (2).
| Formula (2): | Number of vials = Infusion volume (mL) × 0.08 |
Determination of Dosing Frequency
- Obtain a baseline plasminogen activity level. If the patient is receiving plasminogen supplementation with fresh frozen plasma, allow for a 7-day washout period before obtaining baseline plasminogen activity level.
- Initiate RYPLAZIM dosing at a frequency of every three days (Q3D).
- Obtain a trough plasminogen activity level approximately 72 hours following the initial dose of RYPLAZIM and prior to the second dose (same time of day as initial dosing)
- a.
- If the plasminogen activity level is < 10%1 above the baseline plasminogen level, change dosing frequency to Q2D;
- b.
- If the plasminogen activity level is ≥ 10 and ≤ 20%1 above baseline, maintain dosing frequency at Q3D;
- c.
- If the plasminogen activity level is > 20%1 above baseline, change dosing frequency to Q4D.
- Maintain dosing frequency as determined above for 12 weeks while treating active lesions
- a.
- If lesions do not resolve by 12 weeks, or there are new or recurrent lesions, increase dosing frequency in one-day increments every 4-8 weeks up to Q2D dosing while reassessing clinical improvement until lesion resolution or until the lesions stabilize without further worsening. If desired clinical change does not occur by 12 weeks, check trough plasminogen activity level.
- 1)
- If the trough plasminogen activity level is ≥ 10%1 above the baseline trough level, consider other treatment options, such as surgical removal of the lesion in addition to plasminogen treatment.
- 2)
- If the trough plasminogen activity level is < 10%1 above the baseline trough level, obtain a second trough plasminogen activity level to confirm. If low plasminogen activity level is confirmed in combination with no clinical efficacy, consider discontinuing plasminogen treatment due to the possibility of neutralizing antibodies [see Neutralizing Antibodies (5.5)].
- b.
- If lesions resolve by 12 weeks, continue at same dosing frequency and monitor for new or recurrent lesions every 12 weeks.
- 1
- Absolute change in plasminogen activity (%)
Preparation and Reconstitution
Prepare RYPLAZIM within 3 hours of administration. Gather the following additional supplies before performing reconstitution and administration:
- One 20-mL syringe per vial of RYPLAZIM for product reconstitution
- 18- to 22-gauge needles for reconstitution and administration
- Sterile Water for Injection, USP (SWFI) (10-mL, 20-mL or 50-mL vials)
- One syringe disc filter per infusion (Baxter Supor® 5micron Syringe Filter or equivalent)
- One (or more) administration syringe(s) (20-mL, 30-mL or 60-mL)
- Alcohol wipes
- Antiseptic surface wipes
- Medical tape
- Butterfly needle or sterile infusion set
- 10 mL normal saline
- Sterile gauze pad
- Bandage
Reconstitution of RYPLAZIM
Determine the number of RYPLAZIM vials needed using Formula (2) [see Dosage and Administration (2.1)]. Check the expiration date of each vial of RYPLAZIM. Discard any expired vials. Allow RYPLAZIM vials to equilibrate to room temperature before reconstitution (at least 15 minutes if stored at 5 °C). Do not refrigerate after reconstitution.
Work on a clean surface and wash hands before performing the following procedures.
- Remove the caps from the RYPLAZIM vials and Sterile Water for Injection, USP (SWFI) vials to expose the central portion of the rubber stoppers.
- Sterilize the surface of the rubber stoppers with alcohol wipes and allow to dry. Do not blow on it.
- Using a 20-mL sterile syringe with a sterile 18- to 22-gauge needle, withdraw 12.5 mL of SWFI for each vial of RYPLAZIM.
Note: If using 10-mL vials of SWFI, each vial of RYPLAZIM requires two 10-mL vials of SWFI. Withdraw 9.0 mL of SWFI from the first 10-mL vial. Discard the first needle, attach a new 18- to 22-gauge sterile needle and withdraw 3.5 mL SWFI from the second 10-mL vial to equal 12.5 mL. Discard the used SWFI vials. Repeat this process for each vial of RYPLAZIM that needs to be reconstituted.
If using a 20-mL or 50-mL vial of SWFI, each vial of RYPLAZIM requires only one vial of SWFI for reconstitution. Discard the used SWFI vials. - Using the same needle and syringe gently and slowly add the 12.5 mL of SWFI to the RYPLAZIM vial, directing the syringe down toward the side of the RYPLAZIM vial to prevent foaming. This should resemble a stream along the side of the vial (Figure 1). Discard the used syringe and needle(s).
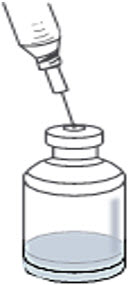
Figure 1
- Gently swirl the vial by rotating it slowly to ensure that the lyophilized powder dissolves fully (Figure 2). Do not shake the vial. RYPLAZIM should fully dissolve within 10 minutes. Discard the vial if RYPLAZIM is not fully dissolved after 10 minutes.
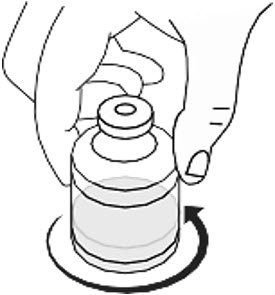
Figure 2
- Observe the reconstituted vials; the solution should be colorless and clear to slightly opalescent. Discard the vial if discoloration or particulate matter are observed.
- Repeat Steps 1 to 6 above to reconstitute each additional vial of RYPLAZIM.
Preparation of RYPLAZIM for Administration
Select an administration syringe of appropriate volume based on the infusion volume calculated using Formula (1) [see Dosage and Administration (2.1)].
Note: A 30-mL syringe can hold up to 2 vials of reconstituted RYPLAZIM and a 60-mL syringe can hold up to 4 vials of reconstituted RYPLAZIM.
Using the selected administration syringe(s) with 18- to 22-gauge needle(s), slowly withdraw the reconstituted RYPLAZIM from each vial to administer the exact infusion volume calculated using Formula (1) [see Dosage and Administration (2.1)].
Do not mix RYPLAZIM with other medications.
Administration
For intravenous use only through a syringe disc filter.
Follow the steps below for infusion:
- One filter is needed per infusion.
- Only administer RYPLAZIM by infusing it into a vein through a syringe disc filter.
- Inspect the solution in the syringe. Do not use if discoloration or particulate matter are observed.
- Administer RYPLAZIM by a separate infusion line. Do not administer RYPLAZIM with other medications.
- Draw 10 mL of normal saline into a different syringe. Push the plunger down to remove any air bubbles.
- Attach a syringe disc filter to the pre-filled syringe of normal saline (from previous step) and the infusion tubing with the butterfly needle. (Figure 3)
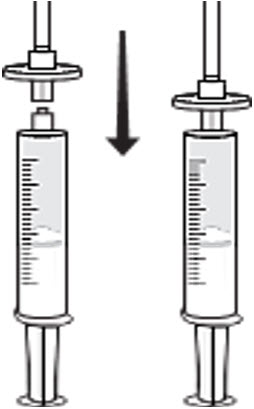
Figure 3
- Inject the normal saline through the syringe disc filter and butterfly needle tubing to remove any air bubbles.
- Remove the normal saline syringe. The syringe disc filter must remain attached to the tubing, as it is required for administration of the RYPLAZIM. Discard the normal saline syringe.
- Attach the administration syringe containing RYPLAZIM to the syringe disc filter that is connected to the butterfly needle tubing.
- Choose a peripheral vein (e.g., antecubital or dorsum of hand). Clean the injection site with a sterile alcohol wipe and allow to dry. Do not blow on it.
- Insert the butterfly infusion set needle in the peripheral vein, and tape in place.
- Infuse the total dose of RYPLAZIM slowly over 10-30 minutes (approximately 5 mL/min). Using a timer (e.g., watch or clock), push the plunger of the syringe approximately 1 mL every 12 seconds. (Figure 4)
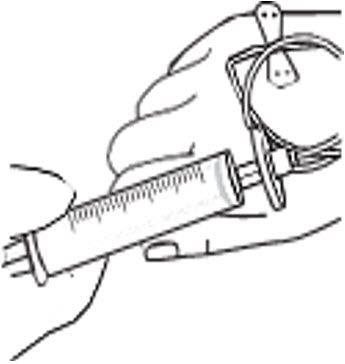
Figure 4
- Discard any open vials, unused solution, and administration equipment following administration.








