Drug Detail:Skyla (Levonorgestrel intrauterine system [ lee-voe-nor-jes-trel-in-tra-ue-ter-ine-sis-tem ])
Generic Name: LEVONORGESTREL 13.5mg
Dosage Form: intrauterine device
Drug Class: Contraceptives Progestins
Dosing Over Time
Skyla contains 13.5 mg of levonorgestrel (LNG) released in vivo at a rate of approximately 14 mcg/day after 24 days. This rate decreases progressively to approximately 6 mcg/day after 1 year and to 5 mcg/day after 3 years. The average in vivo release rate of LNG is approximately 8 mcg/day over the first year of use and 6 mcg/day over a period of 3 years. [See Clinical Pharmacology (12.3).]
Skyla must be removed by the end of the third year and can be replaced at the time of removal with a new Skyla if continued contraceptive protection is desired.
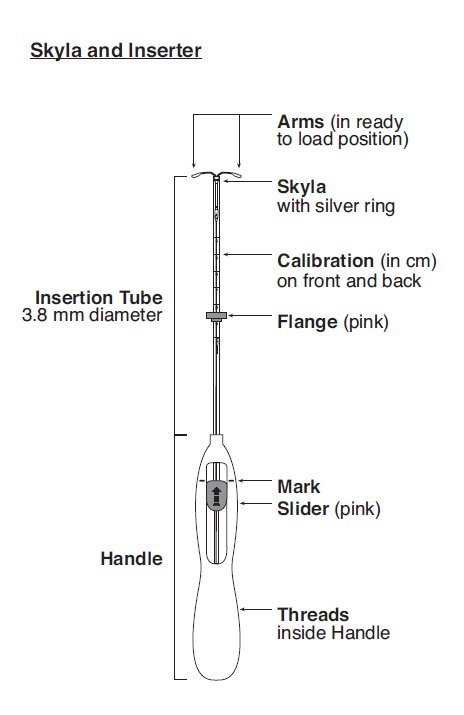
Skyla can be physically distinguished from other intrauterine systems (IUSs) by the combination of the visibility of the silver ring on ultrasound and the brown color of the removal threads.
Skyla is supplied in a sterile package within an inserter that enables single-handed loading (see Figure 1). Do not open the package until required for insertion [see Description (11.2)]. Do not use if the seal of the sterile package is broken or appears compromised. Use strict aseptic techniques throughout the insertion procedure [see Warnings and Precautions (5.3)].
Insertion Instructions
- •
- Obtain a complete medical and social history to determine conditions that might influence the selection of a levonorgestrel-releasing intrauterine system (LNG IUS) for contraception. If indicated, perform a physical examination, and appropriate tests for any forms of genital or other sexually transmitted infections. [See Contraindications (4) and Warnings and Precautions (5.10).] Because irregular bleeding/spotting is common during the first months of Skyla use, exclude endometrial pathology (polyps or cancer) prior to the insertion of Skyla in women with persistent or uncharacteristic bleeding [see Warnings and Precautions (5.8)].
- •
- Follow the insertion instructions exactly as described to ensure proper placement and avoid premature release of Skyla from the inserter. Once released, Skyla cannot be re-loaded.
- •
- Skyla should be inserted by a trained healthcare provider. Healthcare providers should become thoroughly familiar with the insertion instructions before attempting insertion of Skyla.
- •
- Insertion may be associated with some pain and/or bleeding or vasovagal reactions (for example, syncope, bradycardia) or with seizure, especially in patients with a predisposition to these conditions. Consider administering analgesics prior to insertion.
Timing of Insertion
|
Starting Skyla in women not currently using hormonal or intrauterine contraception |
|
|
Switching to Skyla from an oral, transdermal or vaginal hormonal contraceptive |
|
|
Switching to Skyla from an injectable progestin contraceptive |
|
|
Switching to Skyla from a contraceptive implant or another IUS |
|
|
Inserting Skyla after first trimester abortion or miscarriage |
|
|
Inserting Skyla after childbirth or second-trimester abortion or miscarriage |
|
|
|
|
Interval insertion following complete involution of the uterus |
|
Tools for Insertion
Note: The inserter provided with Skyla (see Figure 1) and the Insertion Procedure described in this section are not applicable for immediate insertion after childbirth or second-trimester abortion or miscarriage. For immediate insertion, remove Skyla from the inserter by first loading (see Figure 2) and then releasing (see Figure 7) Skyla from the inserter, and insert according to accepted practice.
Preparation for insertion
- •
- Exclude pregnancy and confirm that there are no other contraindications to the use of Skyla.
- •
- Check expiration date of Skyla prior to initiating insertion.
- •
- With the patient comfortably in lithotomy position, do a bimanual exam to establish the size, shape and position of the uterus.
- •
- Gently insert a speculum to visualize the cervix.
- •
- Thoroughly cleanse the cervix and vagina with a suitable antiseptic solution.
- •
- Prepare to sound the uterine cavity. Grasp the upper lip of the cervix with a tenaculum forceps and gently apply traction to stabilize and align the cervical canal with the uterine cavity. Perform a paracervical block if needed. If the uterus is retroverted, it may be more appropriate to grasp the lower lip of the cervix. The tenaculum should remain in position and gentle traction on the cervix should be maintained throughout the insertion procedure.
- •
- Gently insert a uterine sound to check the patency of the cervix, measure the depth of the uterine cavity in centimeters, confirm cavity direction, and detect the presence of any uterine anomaly. If you encounter difficulty or cervical stenosis, use dilatation, and not force, to overcome resistance. If cervical dilatation is required, consider using a paracervical block.
Insertion Procedure
Proceed with insertion only after completing the above steps and ascertaining that the patient is appropriate for Skyla. Ensure use of aseptic technique throughout the entire procedure.
Step 1–Opening of the package
- •
- Open the package (Figure 1). The contents of the package are sterile.
- •
- Using sterile gloves, lift the handle of the sterile inserter and remove from the sterile package.
Step 2–Load Skyla into the insertion tube
- •
- Push the slider forward as far as possible in the direction of the arrow thereby moving the insertion tube over the Skyla T-body to load Skyla into the insertion tube (Figure 2). The tips of the arms will meet to form a rounded end that extends slightly beyond the insertion tube.
- •
- Maintain forward pressure with your thumb or forefinger on the slider. DO NOT move the slider downward at this time as this may prematurely release the threads of Skyla. Once the slider is moved below the mark, Skyla cannot be re-loaded.
Step 3–Setting the Flange
- •
- Holding the slider in this forward position, set the upper edge of the flange to correspond to the uterine depth (in centimeters) measured during sounding (Figure 3).
Step 4–Skyla is now ready to be inserted
- •
- Continue holding the slider in this forward position. Advance the inserter through the cervix until the flange is approximately 1.5–2 cm from the cervix and then pause (Figure 4).
Do not force the inserter. If necessary, dilate the cervical canal.
Step 5–Open the arms
- •
- While holding the inserter steady, move the slider down to the mark to release the arms of Skyla (Figure 5). Wait 10 seconds for the horizontal arms to open completely.
Step 6–Advance to fundal position
Advance the inserter gently towards the fundus of the uterus until the flange touches the cervix. If you encounter fundal resistance do not continue to advance. Skyla is now in the fundal position (Figure 6). Fundal positioning of Skyla is important to prevent expulsion.
Step 7–Release Skyla and withdraw the inserter
- •
- Holding the entire inserter firmly in place, release Skyla by moving the slider all the way down (Figure 7).
- •
- Continue to hold the slider all the way down while you slowly and gently withdraw the inserter from the uterus.
- •
- Using a sharp, curved scissor, cut the threads perpendicular, leaving about 3 cm visible outside of the cervix [cutting threads at an angle may leave sharp ends (Figure 8)]. Do not apply tension or pull on the threads when cutting to prevent displacing Skyla.
.
- Skyla insertion is now complete. Prescribe analgesics, if indicated. Record the Skyla lot number in the patient records.
Important information to consider during or after insertion
- •
- If you suspect that Skyla is not in the correct position, check placement (for example, using transvaginal ultrasound). Remove Skyla if it is not positioned completely within the uterus. Do not reinsert a removed Skyla.
If there is clinical concern, exceptional pain or bleeding during or after insertion, take appropriate steps (such as physical examination and ultrasound) immediately to exclude perforation.
Patient Follow-up
Reexamine and evaluate patients 4 to 6 weeks after insertion and once a year thereafter, or more frequently if clinically indicated.
Removal of Skyla
Timing of Removal
- •
- Skyla should not remain in the uterus after 3 years.
- •
- If pregnancy is not desired, remove Skyla during the first 7 days of the menstrual cycle, provided the woman is still experiencing regular menses. If removal will occur at other times during the cycle or the woman does not experience regular menses, she is at risk of pregnancy, start a new contraceptive method a week prior to removal for these women. [See Dosage and Administration (2.5).]
Removal Procedure
- •
- Remove Skyla by applying gentle traction on the threads with forceps (Figure 9).
- •
- If the threads are not visible, determine location of Skyla by ultrasound [see Warnings and Precautions (5.10)].
- •
- If Skyla is found to be in the uterine cavity on ultrasound exam, it may be removed using a narrow forceps, such as an alligator forceps. This may require dilation of the cervical canal. After removal of Skyla, examine the system to ensure that it is intact.
Removal may be associated with some pain and/or bleeding or vasovagal reactions (for example, syncope, bradycardia) or seizure, especially in patients with a predisposition to these conditions.
Continuation of Contraception after Removal
- •
- If pregnancy is not desired and if a woman wishes to continue using Skyla, a new system can be inserted immediately after removal any time during the cycle.
- •
- If a patient with regular cycles wants to start a different contraceptive method, time removal and initiation of the new method to ensure continuous contraception. Either remove Skyla during the first 7 days of the menstrual cycle and start the new method immediately thereafter or start the new method at least 7 days prior to removing Skyla if removal is to occur at other times during the cycle.
If a patient with irregular cycles or amenorrhea wants to start a different contraceptive method, start the new method at least 7 days before removal.