Drug Detail:Spravato nasal spray (Esketamine (nasal) [ es-ket-a-meen ])
Generic Name: ESKETAMINE HYDROCHLORIDE 28mg in 0.2mL
Dosage Form: nasal solution
Drug Class: Miscellaneous antidepressants
Important Considerations Prior to Initiating and During Therapy
SPRAVATO must be administered under the direct supervision of a healthcare provider. A treatment session consists of nasal administration of SPRAVATO and post-administration observation under supervision.
Blood Pressure Assessment Before and After Treatment
- Assess blood pressure prior to dosing with SPRAVATO [see Warnings and Precautions (5.6)] .
- If baseline blood pressure is elevated (e.g., >140 mmHg systolic, >90 mmHg diastolic), consider the risks of short term increases in blood pressure and benefit of SPRAVATO treatment [see Warnings and Precautions (5.6)]. Do not administer SPRAVATO if an increase in blood pressure or intracranial pressure poses a serious risk [see Contraindications (4)] .
- After dosing with SPRAVATO, reassess blood pressure at approximately 40 minutes (which corresponds with the C max) and subsequently as clinically warranted .
- If blood pressure is decreasing and the patient appears clinically stable for at least two hours, the patient may be discharged at the end of the post-dose monitoring period; if not, continue to monitor [see Warnings and Precautions (5.6)].
Food and Liquid Intake Recommendations Prior to Administration
Because some patients may experience nausea and vomiting after administration of SPRAVATO [see Adverse Reactions (6.1)], advise patients to avoid food for at least 2 hours before administration and to avoid drinking liquids at least 30 minutes prior to administration.
Treatment-Resistant Depression
Administer SPRAVATO in conjunction with an oral antidepressant (AD).
The recommended dosage of SPRAVATO for the treatment of TRD in adults is shown in Table 1. Dosage adjustments should be made based on efficacy and tolerability. Evidence of therapeutic benefit should be evaluated at the end of the induction phase to determine need for continued treatment.
|
||
| Adults | ||
| Induction Phase | Weeks 1 to 4: | Day 1 starting dose: 56 mg |
| Administer twice per week | Subsequent doses: 56 mg or 84 mg | |
| Maintenance Phase | Weeks 5 to 8: | |
| Administer once weekly | 56 mg or 84 mg | |
| Week 9 and after: | ||
| Administer every 2 weeks or once weekly * | 56 mg or 84 mg | |
Depressive Symptoms in Patients with Major Depressive Disorder with Acute Suicidal Ideation or Behavior
Administer SPRAVATO in conjunction with an oral antidepressant (AD).
The recommended dosage of SPRAVATO for the treatment of depressive symptoms in adults with MDD with acute suicidal ideation or behavior is 84 mg twice per week for 4 weeks. Dosage may be reduced to 56 mg twice per week based on tolerability. After 4 weeks of treatment with SPRAVATO, evidence of therapeutic benefit should be evaluated to determine need for continued treatment. The use of SPRAVATO, in conjunction with an oral antidepressant, beyond 4 weeks has not been systematically evaluated in the treatment of depressive symptoms in patients with MDD with acute suicidal ideation or behavior.
Administration Instructions
SPRAVATO is for nasal use only. The nasal spray device delivers a total of 28 mg of esketamine. To prevent loss of medication, do not prime the device before use. Use 2 devices (for a 56 mg dose) or 3 devices (for an 84 mg dose), with a 5-minute rest between use of each device. Follow these administration instructions and read the Instructions for Use before administration:
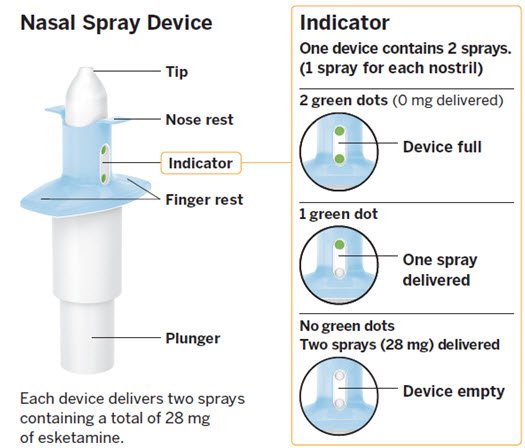
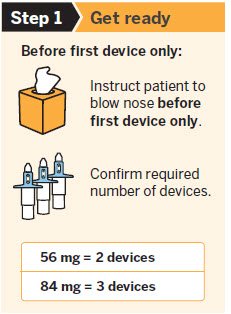
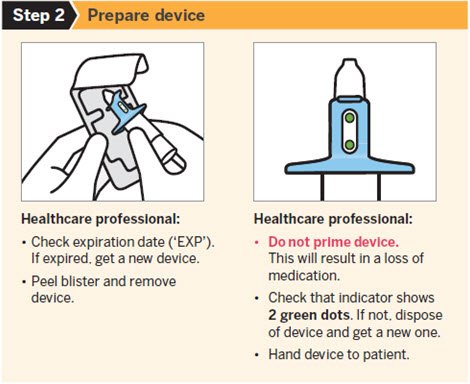
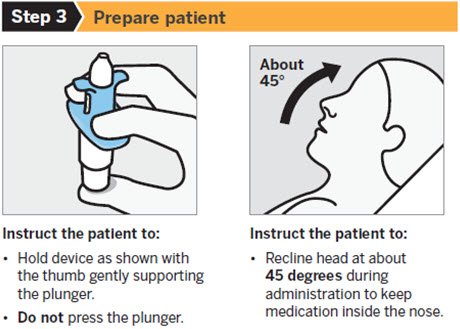

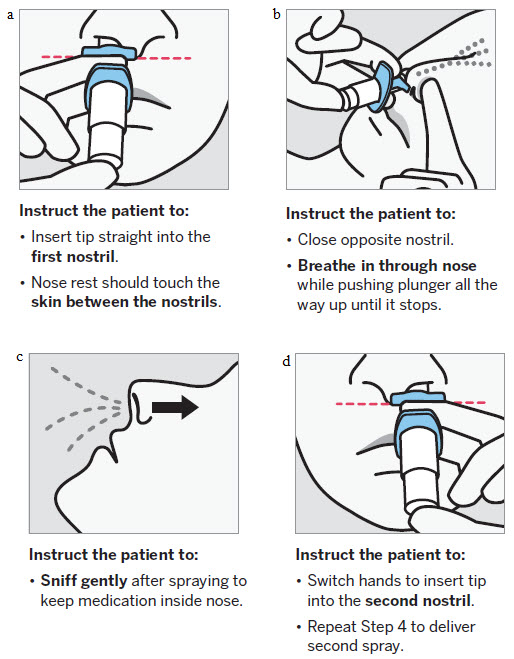
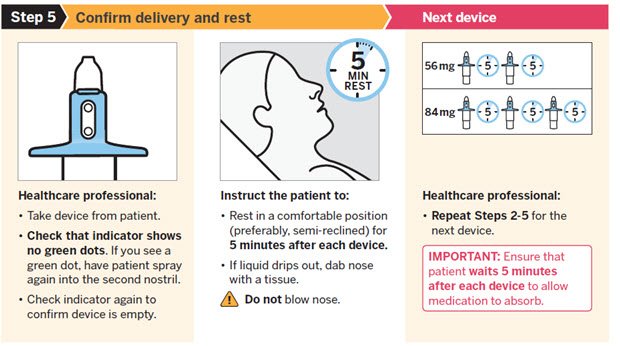

Post-Administration Observation
During and after SPRAVATO administration at each treatment session, observe the patient for at least 2 hours until the patient is safe to leave [see Warnings and Precautions (5.1, 5.2, 5.6, 5.8)] . Before SPRAVATO administration, instruct patients not to engage in potentially hazardous activities, such as driving a motor vehicle or operating machinery, until the next day after a restful sleep.
Missed Treatment Session(s)
If a patient misses treatment session(s), provided there is no worsening of their depressive symptoms, the patient should continue the current dosing schedule.
For patients who miss treatment session(s) during maintenance treatment and have worsening of depression symptoms, per clinical judgement, consider returning to the previous dosing schedule (e.g., if doses missed during weekly dosing, revert to twice weekly dosing).




