Drug Detail:Testim (Testosterone topical [ tes-tos-ter-one-top-i-kal ])
Generic Name: TESTOSTERONE 50mg in 5g
Dosage Form: gel
Drug Class: Androgens and anabolic steroids
Prior to initiating TESTIM, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
Dosing and Dose Adjustment
The recommended starting dose of TESTIM is 50 mg of testosterone (one tube) applied once daily (preferably in the morning) to clean, dry intact skin of the shoulders and/or upper arms.
Dose Adjustment
To ensure proper dosing, serum testosterone concentrations should be measured. Morning, pre-dose serum testosterone concentrations should be measured approximately 14 days after initiation of therapy to ensure proper serum testosterone concentrations are achieved. If the serum testosterone concentration is below the normal range (300 ng/dL to 1,000 ng/dL), the daily TESTIM dose may be increased from 50 mg testosterone (one tube) to 100 mg testosterone (two tubes) once daily.
The maximum recommended dose of TESTIM is 100 mg once daily.
The application site and dose of TESTIM are not interchangeable with other topical testosterone products.
Administration Instructions
Upon opening the tube the entire contents should be squeezed into the palm of the hand and immediately applied to the shoulders and/or upper arms (area of application should be limited to the area that will be covered by the patient’s short sleeve T-shirt (see figure below). Do not apply TESTIM to the genitals or to the abdomen.
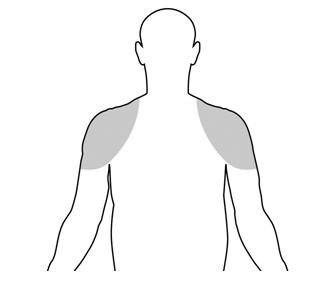
Application sites should be allowed to dry for a few minutes prior to dressing. Hands should be washed thoroughly with soap and water after TESTIM has been applied. Avoid fire, flame or smoking during the application of TESTIM until the TESTIM has dried [see Warnings and Precautions (5.2), (5.16)].
In order to prevent transfer to another person, wear clothing to cover the application sites. If direct skin-to-skin contact with another person is anticipated, the application sites must be washed thoroughly with soap and water [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
The patient should avoid swimming or showering or washing the administration site for a minimum of 2 hours after application [see Clinical Pharmacology (12.3)].
Strict adherence to the following precautions is advised in order to minimize the potential for secondary exposure to testosterone from TESTIM-treated skin:
- Children and women should avoid contact with unwashed or unclothed application site(s) of men using TESTIM.
- TESTIM should only be applied to the upper arms and shoulders. The area of application should be limited to the area that will be covered by a short sleeve T-shirt.
- Patients should wash their hands with soap and water immediately after applying TESTIM.
- Patients should cover the application site(s) with clothing (e.g., a T-shirt) after the gel has dried.
- Prior to situations in which direct skin-to-skin contact is anticipated, patients should wash the application site(s) thoroughly with soap and water to remove any testosterone residue.
- In the event that unwashed or unclothed skin to which TESTIM has been applied comes in direct contact with the skin of another person, the general area of contact on the other person should be washed with soap and water as soon as possible.




