Drug Detail:Tri-lo-sprintec (Ethinyl estradiol and norgestimate [ eth-i-nil-es-tra-dye-ol-and-nor-jes-ti-mate ])
Generic Name: NORGESTIMATE 0.18mg, ETHINYL ESTRADIOL 0.025mg; NORGESTIMATE 0.215mg, ETHINYL ESTRADIOL 0.025mg; NORGESTIMATE 0.25mg, ETHINYL ESTRADIOL 0.025mg;
Dosage Form: tablets
Drug Class: Contraceptives
How to Start Tri-Lo-Sprintec
Tri-Lo-Sprintec is dispensed in a blister pack tablet dispenser [see How Supplied/Storage and Handling (16)]. Tri-Lo-Sprintec may be started using either a Day 1 start or a Sunday start (see Table 1). For the first cycle of a Sunday Start regimen, an additional method of contraception should be used until after the first 7 consecutive days of administration.
How to Take Tri-Lo-Sprintec
|
Table 1: Instructions for Administration of Tri-Lo-Sprintec |
|
|
Starting COCs in women not currently using hormonal contraception (Day 1 Start or Sunday Start) Important: Consider the possibility of ovulation and conception prior to initiation of this product. Tablet Color:
|
Day 1 Start:
Sunday Start:
|
|
Switching to Tri-Lo-Sprintec from another oral contraceptive |
Start on the same day that a new pack of the previous oral contraceptive would have started. |
|
Switching from another contraceptive method to Tri-Lo-Sprintec |
Start Tri-Lo-Sprintec: |
|
|
|
|
|
|
|
|
|
|
|
Complete instructions to facilitate patient counseling on proper tablet usage are located in the FDA-Approved Patient Labeling. |
|
Starting Tri-Lo-Sprintec after Abortion or Miscarriage
First-trimester
- After a first-trimester abortion or miscarriage, Tri-Lo-Sprintec may be started immediately. An additional method of contraception is not needed if Tri-Lo-Sprintec is started immediately.
- If Tri-Lo-Sprintec is not started within 5 days after termination of the pregnancy, the patient should use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of her first cycle pack of Tri-Lo-Sprintec.
Second-trimester
- Do not start until 4 weeks after a second-trimester abortion or miscarriage, due to the increased risk of thromboembolic disease. Start Tri-Lo-Sprintec, following the instructions in Table 1 for Day 1 or Sunday start, as desired. If using Sunday start, use additional non-hormonal contraception (such as condoms and spermicide) for the first seven days of the patient’s first cycle pack of Tri-Lo-Sprintec. [See Contraindications (4), Warnings and Precautions (5.1), and FDA-Approved Patient Labeling.]
Starting Tri-Lo-Sprintec after Childbirth
- Do not start until 4 weeks after delivery, due to the increased risk of thromboembolic disease. Start contraceptive therapy with Tri-Lo-Sprintec following the instructions in Table 1 for women not currently using hormonal contraception.
- Tri-Lo-Sprintec is not recommended for use in lactating women [see Use in Specific Populations (8.3)].
- If the woman has not yet had a period postpartum, consider the possibility of ovulation and conception occurring prior to use of Tri-Lo-Sprintec. [See Contraindications (4), Warnings and Precautions (5.1), Use in Specific Populations (8.1 and 8.3), and FDA-Approved Patient Labeling].
How to Use the Blister Cards
There are two ways to start taking birth control pills, Sunday Start or Day 1 Start. Your healthcare professional will tell you which to use.
- Pick the Days of the Week Sticker that starts the first day of your period. (This is the day you begin bleeding or spotting, even if it is midnight when bleeding begins.) When you have picked the right sticker, throw away the others and place the sticker on the blister card over the pre-printed days of the week and make sure it lines up with the pills.
- Your blister package consists of three parts, the foil pouch, wallet, and a blister pack containing 28 individually sealed pills. Note that the pills are arranged in four numbered rows of 7 pills, with the pre-printed days of the week printed above them. There are 7 gray “active” pills, 7 light blue “active” pills, 7 blue “active” pills, and 7 white “reminder” pills. Refer to the sample of the blister card below:
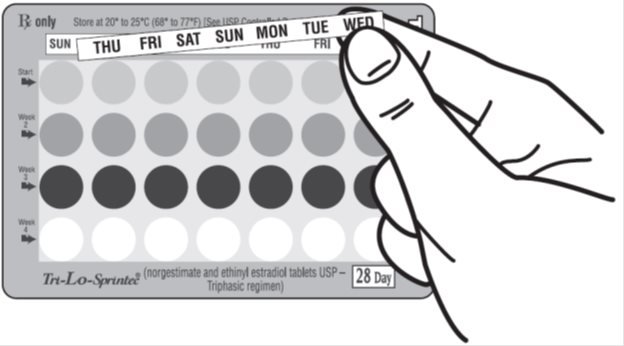
- After taking the last white pill, start a new blister card the very next day no matter when your period started. You will be taking a pill every day without interruption. Anytime you start the pills later than directed, protect yourself by using another method of birth control until you have taken a pill a day for seven consecutive days. After taking the last white pill, start taking the first gray pill from the blister card the very next day.
- Take the pills in each new package as before. Start with the gray pill on row #1 and take one pill each day, left to right, until the last white pill has been taken.
Three Ways to Remember in What Order to Take the Pills
- Follow the sticker with the days of the week (placed above the pills).
- Always go from left to right.
- Always finish all your pills.
Missed Tablets
|
Table 2: Instructions for Missed Tri-Lo-Sprintec Tablets |
|
|
Take the tablet as soon as possible. Continue taking one tablet a day until the pack is finished. |
|
Take the two missed tablets as soon as possible and the next two active tablets the next day. Continue taking one tablet a day until the pack is finished. Additional non-hormonal contraception (such as condoms and spermicide) should be used as back‑up if the patient has sex within 7 days after missing tablets. |
|
Day 1 start: Throw out the rest of the pack and start a new pack that same day. Sunday start: Continue taking one tablet a day until Sunday, then throw out the rest of the pack and start a new pack that same day. Additional non-hormonal contraception (such as condoms and spermicide) should be used as back-up if the patient has sex within 7 days after missing tablets. |
Advice in Case of Gastrointestinal Disturbances
In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting or diarrhea occurs within 3 to 4 hours after taking an active tablet, handle this as a missed tablet [see FDA-Approved Patient Labeling].





