Drug Detail:Viramune (Nevirapine [ ne-vye-ra-peen ])
Generic Name: NEVIRAPINE 400mg
Dosage Form: tablet, extended release
Drug Class: NNRTIs
General Dosing Considerations
- VIRAMUNE XR tablets must be swallowed whole and must not be chewed, crushed, or divided.
- Children should be assessed for their ability to swallow tablets before prescribing VIRAMUNE XR tablets.
- VIRAMUNE XR can be taken with or without food.
Adult Patients
Patients not currently taking immediate-release VIRAMUNE
Patients must initiate therapy with one 200 mg tablet of immediate-release VIRAMUNE daily for the first 14 days in combination with other antiretroviral agents. The 14-day lead-in period with VIRAMUNE 200 mg daily dosing must be strictly followed (the lead-in period has been observed to decrease the incidence of rash), followed by one 400 mg tablet of VIRAMUNE XR once daily [see Dosage and Administration (2.5) and Warnings and Precautions (5.2)]. If rash persists beyond the 14-day lead-in period with immediate-release VIRAMUNE, do not begin dosing with VIRAMUNE XR. The lead-in dosing with 200 mg once daily immediate-release VIRAMUNE should not be continued beyond 28 days, at which point an alternative regimen should be sought.
Switching patients from immediate-release VIRAMUNE to VIRAMUNE XR
Patients already on a regimen of immediate-release VIRAMUNE twice daily in combination with other antiretroviral agents can be switched to VIRAMUNE XR 400 mg once daily without the 14-day lead-in period. Patients already on a regimen of immediate-release VIRAMUNE twice daily who switch to VIRAMUNE XR therapy should continue with their ongoing clinical and laboratory monitoring.
Pediatric Patients
VIRAMUNE XR in pediatric patients is dosed based on body surface area (BSA) calculated using the Mosteller formula. All pediatric patients must initiate therapy with immediate-release VIRAMUNE (as 150 mg/m2 of VIRAMUNE Oral Suspension or as VIRAMUNE tablets), at a dose not to exceed 200 mg per day, administered once daily for the first 14 days. This lead-in period should be used because it has been demonstrated to reduce the frequency of rash. This lead-in period is not required if the patient is already on a regimen of twice daily immediate-release formulation in combination with other antiretroviral agents.
The recommended oral dose of VIRAMUNE XR for pediatric patients with a BSA of 1.17 m2 or greater is 400 mg following the lead-in period with immediate-release VIRAMUNE. The total daily dose should not exceed 400 mg for any patient.
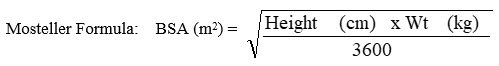
Monitoring of Patients
Intensive clinical and laboratory monitoring, including liver enzyme tests, is essential at baseline and during the first 18 weeks of treatment with nevirapine. The optimal frequency of monitoring during this period has not been established. Some experts recommend clinical and laboratory monitoring more often than once per month, and in particular, would include monitoring of liver enzyme tests prior to beginning the 14-day lead-in period with immediate-release VIRAMUNE, prior to initiation of VIRAMUNE XR, and at two weeks after initiation of VIRAMUNE XR therapy. After the initial 18-week period, frequent clinical and laboratory monitoring should continue throughout VIRAMUNE XR treatment [see Warnings and Precautions (5)]. In some cases, hepatic injury has progressed despite discontinuation of treatment.
Patients already on a regimen of immediate-release VIRAMUNE twice daily who switch to VIRAMUNE XR once daily should continue with their ongoing clinical and laboratory monitoring.
Dosage Adjustment
Patients with Rash
Discontinue nevirapine if a patient experiences severe rash or any rash accompanied by constitutional findings [see Warnings and Precautions (5.2)]. Do not initiate therapy with VIRAMUNE XR if a patient experiences mild to moderate rash without constitutional symptoms during the 14-day lead-in period of immediate-release VIRAMUNE until the rash has resolved [see Warnings and Precautions (5.2)]. The total duration of the once daily lead-in dosing period should not exceed 28 days at which point an alternative regimen should be sought.
Patients with Hepatic Events
If a clinical (symptomatic) hepatic event occurs, permanently discontinue nevirapine. Do not restart nevirapine after recovery [see Warnings and Precautions (5.1)].
Patients with Dose Interruption
For patients who interrupt VIRAMUNE XR dosing for more than 7 days, restart the recommended lead-in dosing with immediate-release VIRAMUNE, using one 200 mg tablet daily for the first 14 days.
Patients with Renal Impairment
Patients with CrCl greater than or equal to 20 mL per min and not requiring dialysis do not require an adjustment in dosing. The pharmacokinetics of nevirapine have not been evaluated in patients with CrCl less than 20 mL per min. An additional 200 mg dose of immediate-release VIRAMUNE following each dialysis treatment is indicated in patients requiring dialysis. Nevirapine metabolites may accumulate in patients receiving dialysis; however, the clinical significance of this accumulation is not known [see Clinical Pharmacology (12.3)]. VIRAMUNE XR has not been studied in patients with renal dysfunction.




