Drug Detail:Zemaira (Alpha 1-proteinase inhibitor [ al-fa-1-pro-tee-nase-in-hib-i-tor ])
Generic Name: .ALPHA.1-PROTEINASE INHIBITOR HUMAN 1000mg in 20mL;
Dosage Form: injection
Drug Class: Miscellaneous respiratory agents
For intravenous use after reconstitution only.
The recommended dose of ZEMAIRA is 60 mg/kg body weight administered once weekly. Dose ranging studies using efficacy endpoints have not been performed with ZEMAIRA or any A1-PI product.
Preparation and Reconstitution
- Check the expiration date on the vial label and carton. Do not use ZEMAIRA after the expiration date.
- Reconstitute prior to use according to the instructions provided below.
- Reconstitute ZEMAIRA using aseptic technique to maintain product sterility.
- Total reconstitution time for a 1g vial should be obtained within 5 minutes.
- Total reconstitution time for a 4g or 5g vial should be obtained within 10 minutes.
- Inspect the reconstituted solution prior to administration. The solution should be clear, colorless to slightly yellow, and free from visible particles.
- Reconstituted ZEMAIRA may be stored at room temperature. Do not freeze the reconstituted solution.
Follow the steps provided below for the preparation and reconstitution of ZEMAIRA:
- 1.
- Ensure that the ZEMAIRA vial and Sterile Water for Injection vial are at room temperature.
- 2.
- Remove the plastic flip-top cap from the Sterile Water for Injection vial.
- 3.
- Wipe the rubber stopper of the Sterile Water for Injection vial with antiseptic solution and allow it to dry.
- 4.
- Open the Mix2Vial® filter transfer set by peeling off the lid (Fig. 1). Do not remove the transfer set from the blister package.
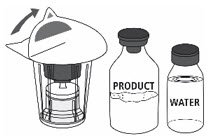
Fig. 1
- 5.
- Place the Sterile Water for Injection vial on an even, clean surface and hold the vial tight. Take the transfer set together with the blister package and vertically pierce the Sterile Water for Injection vial with the blue tip of the transfer set (Fig. 2).
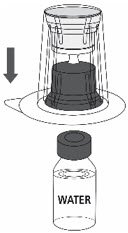
Fig. 2
- 6.
- Carefully remove the blister package from the transfer set by holding at the rim, and pulling vertically upwards. Make sure that you only pull away the blister package and not the transfer set (Fig. 3).
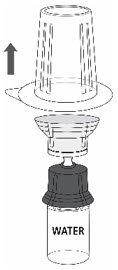
Fig. 3
- 7.
- Remove the plastic flip-top cap from the ZEMAIRA vial.
- 8.
- Wipe the rubber stopper of the ZEMAIRA vial with antiseptic solution and allow it to dry.
- 9.
- Place the ZEMAIRA vial on an even and firm surface. Invert the Sterile Water for Injection vial with the transfer set attached and vertically pierce the ZEMAIRA vial with the clear tip of the transfer set (Fig. 4). The Sterile Water for Injection will automatically flow into the ZEMAIRA vial.
Note: Ensure all water has transferred into the ZEMAIRA vial.
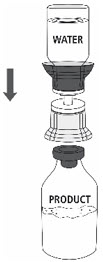
Fig. 4
- 10.
- Follow steps below to remove entire transfer set from ZEMAIRA vial:
- With one hand tightly grasp the ZEMAIRA vial as shown in Fig. 5.
- With the other hand tightly grasp Sterile Water for Injection vial and blue transfer set.
- Bend the entire transfer set to the side until it disconnects from the ZEMAIRA vial (Fig. 5).
- Discard the Sterile Water for Injection vial with the entire transfer set.
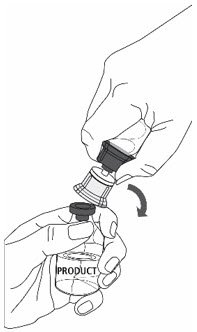
Fig. 5
- 11.
- Gently swirl the ZEMAIRA vial until the powder is completely dissolved (Fig. 6). DO NOT SHAKE. Take care not to touch the rubber vial stopper.

Fig. 6
If more than 1 vial of ZEMAIRA is needed to achieve the required dose, use aseptic technique to transfer the reconstituted solution from the vials into the administration container (e.g., empty intravenous bag or glass bottle).
Administration
For intravenous use only.
- Do not mix ZEMAIRA with other medicinal products; administer ZEMAIRA through a separate dedicated infusion line.
- Perform a visual inspection of the reconstituted solution. The solution should be clear, colorless to slightly yellow, and free from visible particles.
- Administer at room temperature within 3 hours after reconstitution.
- Filter the reconstituted solution during administration. To ensure proper filtration of ZEMAIRA, use an intravenous administration set with a suitable 5 micron infusion filter (not supplied).
- Administer ZEMAIRA intravenously at a rate of approximately 0.08 mL/kg/min as determined by the response and comfort of the patient. The recommended dosage of 60 mg/kg body weight will take approximately 15 minutes to infuse.
- Monitor closely the infusion rate and the patient's clinical state, including vital signs, throughout the infusion. Slow or stop the infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a lower rate that is comfortable for the patient.
- ZEMAIRA is for single dose only. Following administration, discard any unused solution and all administration equipment in an appropriate manner as per local requirements.









