Drug Detail:Zynrelef (Bupivacaine and meloxicam [ byoo-piv-a-kane-and-mel-oks-i-kam ])
Generic Name: BUPIVACAINE 400mg in 14mL, MELOXICAM 12mg in 14mL
Dosage Form: solution
Drug Class: Analgesic combinations
Important Dosage and Administration Information
- ZYNRELEF is intended for single-dose administration only.
- As there is a potential risk of severe, life-threatening adverse reactions associated with the administration of bupivacaine, ZYNRELEF should be administered in a setting where trained personnel and equipment are available to promptly treat patients who show evidence of neurologic or cardiac toxicity [see Overdosage (10)].
- The toxic effects of local anesthetics are additive. Avoid additional use of local anesthetics within 96 hours following administration of ZYNRELEF.
- Avoid intravascular administration of ZYNRELEF. Convulsions and cardiac arrest have occurred following accidental intravascular injection of bupivacaine and other amide-containing products.
- Limit exposure to articular cartilage due to the potential risk of chondrolysis [see Warnings and Precautions (5.10)].
- The safety of concomitant administration of ZYNRELEF and other NSAID medications has not been evaluated. If additional NSAID medication is indicated in the post-operative period, monitor patients for signs and symptoms of NSAID toxicity [see Clinical Pharmacology (12.3)].
- ZYNRELEF is a viscous solution supplied as a kit consisting of a single-dose glass vial, and the following sterile components: Luer Lock syringe(s), a vented vial spike, Luer Lock cone-shaped applicator(s), and syringe tip cap(s). ZYNRELEF should only be prepared and administered with the components provided in the ZYNRELEF kit. See the ZYNRELEF Instructions for Use included in the kit for complete administration instructions with illustrations.
- The contents of the ZYNRELEF vial are sterile. The vial exterior is not sterile. Follow your facility's standard operating procedures regarding aseptic drug preparation.
- Each ZYNRELEF vial contains overfill to compensate for residual amounts that remain in the vial, vented vial spike, Luer lock applicator, and syringe(s) during drug withdrawal and administration.
- ZYNRELEF is applied without a needle into the surgical site following final irrigation and suctioning, and prior to suturing of each layer, when multiple tissue layers are involved.
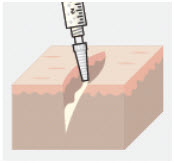
- When ZYNRELEF comes in contact with moisture in the tissues, it becomes more viscous, allowing it to stay in place.
- ZYNRELEF does not degrade sutures. When tying knots with monofilament sutures, contact with ZYNRELEF may cause knots to loosen or untie due to the viscosity of ZYNRELEF. In vitro studies showed an increase in elasticity with monofilament sutures exposed to ZYNRELEF with unknown clinical significance. Minimize administration of ZYNRELEF near the incision line and wipe off excess ZYNRELEF from the skin prior to suturing. Three (3) or more knots ending in a multi-throw knot (e.g. a Surgeon's knot) are recommended with monofilament sutures. Braided or barbed sutures are recommended, especially for closure of deeper layers.
- ZYNRELEF should not be administered via the following routes.–Epidural–Intrathecal–Intravascular or intra-articular–Regional nerve blocks–Pre-incisional or pre-procedural locoregional anesthetic techniques.
Preparation Instructions
- ZYNRELEF is a clear, pale yellow to yellow, viscous liquid. Visually inspect the ZYNRELEF vial for particulate matter and discoloration. Obtain a new vial if particulate matter or discoloration is observed.
- Prepare vial for filling of syringe(s) by attaching vented vial spike. Prepare syringe by filling with air then attach to vented vial spike.
- Invert to allow product to fill the vial neck and push air into vial. Withdraw dose of ZYNRELEF into syringe. (The dose volume takes into account the potential residual volume in the components.)
Nominal Dose of Bupivacaine / Meloxicam (mg/mg) Number of Syringes and LLAs* Per Dose Volume to be Withdrawn (mL) - *
- LLA: Luer lock cone-shaped applicator
60 / 1.8 1 2.3 (using 3 mL syringe provided) 200 / 6 1 7 (using 12 mL syringe provided) 300 / 9 1 10.5 (using 12 mL syringe provided) 400 / 12 2 14 (using two 12 mL syringes provided, 7 mL ZYNRELEF per syringe) - Repeat steps 1-3 for more than one syringe.
- Prepare product immediately prior to use and apply syringe tip cap until product delivery.
Administration Instructions
Before administration, remove the syringe tip cap and attach the Luer lock cone-shaped applicator to the syringe.
- Using the Luer lock cone-shaped applicator attached to the syringe, apply ZYNRELEF to the tissues within the surgical site as follows:
- a.
- For foot and ankle surgical procedures, apply ZYNRELEF to the proximal and distal ends (i.e., beyond the boney repair) of the wound.
- b.
- For small-to-medium open abdominal surgical procedures, close the peritoneum (if applicable), then apply ZYNRELEF above and below the fascial repair.
- c.
- For lower extremity total joint arthroplasty surgical procedures, apply ZYNRELEF directly to the joint capsule, the anteromedial tissues and periosteum, and the anterolateral tissues and periosteum after placement of the components.
- Only apply ZYNRELEF to the tissue layers below the skin incision and not directly onto the subdermal layer or the skin. Minimize administration of ZYNRELEF near the incision line.
- Use only the amount necessary to coat the tissues, such that ZYNRELEF does not leak from the surgical wound after closure. Wipe off excess ZYNRELEF from the skin prior to or during closure of the wound.
Dosing Instructions
As a general guidance in selecting the proper dosing of ZYNRELEF, the following examples of dosing are provided:
- For foot and ankle surgical procedures, such as bunionectomy: up to 2.3 mL to deliver 60 mg of bupivacaine and 1.8 mg of meloxicam [see Clinical Studies (14.1)].
- For small-to-medium open abdominal surgical procedures, such as open inguinal herniorrhaphy: up to 10.5 mL to deliver 300 mg of bupivacaine and 9 mg of meloxicam [see Clinical Studies (14.2)].
- For lower extremity total joint arthroplasty surgical procedures, such as total knee arthroplasty: up to 14 mL to deliver 400 mg of bupivacaine and 12 mg of meloxicam [see Clinical Studies (14.3)].
Compatibility Considerations
- Do not dilute ZYNRELEF.
- ZYNRELEF is a nonaqueous solution. It cannot be mixed with water, saline, or other local anesthetics as the product will become more viscous and difficult to administer.
- When a topical antiseptic such as povidone iodine (e.g., Betadine®) is applied, the site should be allowed to dry before a local anesthetic, including ZYNRELEF, is administered into the site.
- When administered in recommended doses and concentrations, ZYNRELEF does not ordinarily produce irritation or tissue damage.
ZYNRELEF is compatible with:
- All components of the ZYNRELEF kit, including syringes, Luer lock cone-shaped applicator, vented vial spike, and syringe tip caps.
- Surgical mesh materials, including polypropylene (Prolene®), Gore-tex, and polyester.
- Silicone membranes.
- Bone cement.
- Metal alloys used in surgical implants.





