Images
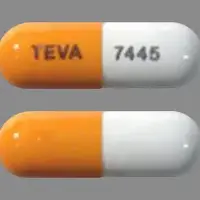
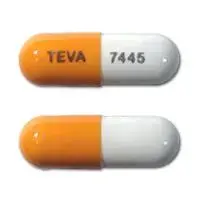
Budesonide (systemic, oral inhalation) (monograph) |
|
| Generic Name: | entocort ec |
| Imprint: | TEVA 7445 |
| Strength: | 3 mg |
| Color: | Peach & White |
| Size: | 20.00 mm |
| Shape: | Capsule-shape |
| Availability: | Prescription only |
| Drug Class: | |
| Pregnancy Category: | C - Risk cannot be ruled out |
| CSA Schedule: | Not a controlled drug |
| Labeler / Supplier: | N/A |
| National Drug Code (NDC): | 00093-7445 (Discontinued) |
| Inactive Ingredients: | Acetyltributyl citrate, Ethylcellulose, Acetic acid, Lactose monohydrate, Methacrylic acid - ethyl acrylate copolymer (1:1) type a, Polysorbate 80, Dimethicone, Sodium hydroxide, Sucrose, Corn starch, Magnesium silicate, Triethyl citrate, FD&C Yellow No. 6, FD&C Red No. 40, Titanium dioxide, D&C Yellow No. 10, FD&C Blue No. 1, FD&C Blue No. 2, Indigotindisulfonate sodium, Shellac, Ferrosoferric oxide, Propylene glycol, |




