Drug Detail:Abecma ( idecabtagene vicleucel)
Drug Class: Miscellaneous antineoplastics
Highlights of Prescribing Information
ABECMA® (idecabtagene vicleucel), suspension for intravenous infusion
Initial U.S. Approval: 2021
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS AND PROLONGED CYTOPENIA
See full prescribing information for complete boxed warning.
- •
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with ABECMA. Do not administer ABECMA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids (2.2, 2.3, 5.1, 6.1).
- •
- Neurologic toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with ABECMA. Provide supportive care and/or corticosteroids as needed (2.2, 2.3, 5.2).
- •
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS), including fatal and life-threatening reactions, occurred in patients following treatment with ABECMA. HLH/MAS can occur with CRS or neurologic toxicities (5.3).
- •
- Prolonged Cytopenia with bleeding and infection, including fatal outcomes following stem cell transplantation for hematopoietic recovery, occurred following treatment with ABECMA (5.7).
- •
- ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS (5.4).
Indications and Usage for Abecma
ABECMA is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody (1).
Abecma Dosage and Administration
For autologous use only. For intravenous use only.
- •
- Do NOT use a leukodepleting filter (2.2).
- •
- Administer a lymphodepleting chemotherapy regimen of cyclophosphamide and fludarabine before infusion of ABECMA (2.2).
- •
- Confirm the patient's identity prior to infusion (2.2).
- •
- Premedicate with acetaminophen and an H1-antihistamine (2.2).
- •
- Avoid prophylactic use of dexamethasone or other systemic corticosteroids (2.2).
- •
- Confirm availability of tocilizumab prior to infusion (2.2, 5.1).
- •
- Dosing of ABECMA is based on the number of chimeric antigen receptor (CAR)-positive T cells (2.1).
- •
- The recommended dose range is 300 to 460 × 106 CAR-positive T cells (2.1).
- •
- Administer ABECMA at a certified healthcare facility (2.2, 5.1, 5.2, 5.3).
Dosage Forms and Strengths
- •
- ABECMA is a cell suspension for intravenous infusion (3).
- •
- A single dose of ABECMA contains a cell suspension of 300 to 460 × 106 CAR-positive T cells in one or more infusion bags (3).
Contraindications
None (4).
Warnings and Precautions
- •
- Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion (5.5).
- •
- Infections: Monitor patients for signs and symptoms of infection; treat appropriately (5.6).
- •
- Prolonged Cytopenias: Patients may exhibit prolonged Grade 3 or higher cytopenias following ABECMA infusion. Monitor blood counts prior to and after ABECMA infusion (5.7).
- •
- Hypogammaglobulinemia: Monitor and consider immunoglobulin replacement therapy (5.8).
- •
- Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with ABECMA, contact Bristol-Myers Squibb at 1-888-805-4555 (5.9).
- •
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving or operating heavy or potentially dangerous machines for at least 8 weeks after ABECMA administration (5.10).
Adverse Reactions/Side Effects
The most common nonlaboratory adverse reactions (incidence greater than or equal to 20%) include CRS, infections – pathogen unspecified, fatigue, musculoskeletal pain, hypogammaglobulinemia, diarrhea, upper respiratory tract infection, nausea, viral infections, encephalopathy, edema, pyrexia, cough, headache, and decreased appetite (6.1).
The most common laboratory adverse reactions (incidence greater than or equal to 50%) include neutropenia, leukopenia, lymphopenia, thrombocytopenia, and anemia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Bristol-Myers Squibb at 1-888-805-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2021
Related/similar drugs
Darzalex, Blenrep, Tecvayli, Revlimid, Velcade, Pomalyst, KyprolisFull Prescribing Information
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, HLH/MAS, AND PROLONGED CYTOPENIA
- •
- Cytokine Release Syndrome (CRS), including fatal or life-threatening reactions, occurred in patients following treatment with ABECMA. Do not administer ABECMA to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1), and Adverse Reactions (6.1)].
- •
- Neurologic toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS. Monitor for neurologic events after treatment with ABECMA. Provide supportive care and/or corticosteroids as needed [see Dosage and Administration (2.2, 2.3) and Warnings and Precautions (5.2)].
- •
- Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS) including fatal and life-threatening reactions, occurred in patients following treatment with ABECMA. HLH/MAS can occur with CRS or neurologic toxicities [see Warnings and Precautions (5.3)].
- •
- Prolonged Cytopenia with bleeding and infection, including fatal outcomes following stem cell transplantation for hematopoietic recovery, occurred following treatment with ABECMA [see Warnings and Precautions (5.7)].
- •
- ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS [see Warnings and Precautions (5.4)].
1. Indications and Usage for Abecma
ABECMA is a B-cell maturation antigen (BCMA)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.
2. Abecma Dosage and Administration
For autologous use only. For intravenous use only.
2.1 Dose
ABECMA is provided as a single dose for infusion containing a suspension of chimeric antigen receptor (CAR)-positive T cells in one or more infusion bags. The recommended dose range is 300 to 460 × 106 CAR-positive T cells.
See the accompanying Release for Infusion Certificate (RFI Certificate) for additional information pertaining to dose [see How Supplied/Storage and Handling (16)].
2.2 Administration
ABECMA is for autologous use only. The patient's identity must match the patient identifiers on the ABECMA cassette(s) and infusion bag(s). Do not infuse ABECMA if the information on the patient-specific label(s) does not match the intended patient.
Preparing Patient for ABECMA Infusion
Confirm the availability of ABECMA prior to starting the lymphodepleting chemotherapy regimen.
Pretreatment
Administer the lymphodepleting chemotherapy regimen: cyclophosphamide 300 mg/m2 intravenously (IV) and fludarabine 30 mg/m2 IV for 3 days.
See the prescribing information of cyclophosphamide and fludarabine for information on dose adjustment in renal impairment.
Administer ABECMA 2 days after completion of lymphodepleting chemotherapy.
Delay the infusion of ABECMA up to 7 days if a patient has any of the following conditions:
- •
- unresolved serious adverse events (especially pulmonary events, cardiac events, or hypotension), including those after preceding chemotherapies
- •
- active infections or inflammatory disorders [see Warnings and Precautions (5.5)].
Premedication
Administer acetaminophen (650 mg orally) and diphenhydramine (12.5 mg IV or 25 to 50 mg orally, or another H1-antihistamine) approximately 30 to 60 minutes before infusion of ABECMA.
Avoid prophylactic use of dexamethasone or other systemic corticosteroids, as the use may interfere with the activity of ABECMA.
Receipt of ABECMA
- •
- ABECMA is shipped directly to the cell laboratory or clinical pharmacy associated with the infusion center in the vapor phase of a liquid nitrogen shipper.
- •
- Confirm the patient's identity with the patient identifiers on the shipper.
- •
- If the patient is not expected to be ready for same-day administration before the shipper expires and the infusion site is qualified for onsite storage, transfer ABECMA to onsite vapor phase of liquid nitrogen storage.
- •
- If the patient is not expected to be ready for same day administration before the shipper expires and the infusion site is not qualified for onsite storage, contact Bristol-Myers Squibb at 1-888-805-4555 to arrange for return shipment.
Preparation of ABECMA for Infusion
- 1.
- Coordinate the timing of ABECMA thaw and infusion. Confirm the infusion time in advance and adjust the start time of the thaw of ABECMA so that it will be available for infusion when the patient is ready.
- 2.
- Prior to thawing the product, confirm that tocilizumab and emergency equipment are available prior to the infusion and during the recovery period.
- 3.
- An ABECMA dose may be contained in one or more patient-specific infusion bag(s). Verify the number of bags received for the indicated dose of ABECMA prior to preparation of ABECMA for infusion.
- 4.
- Confirm patient identity: Prior to preparation of ABECMA, match the patient's identity with the patient identifiers on the ABECMA cassette(s), infusion bag(s), and the RFI Certificate.
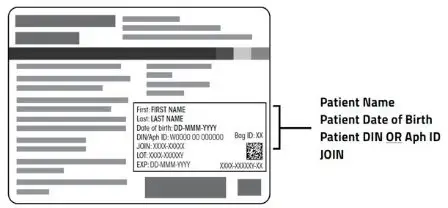
Note: The patient identifier number may be preceded by the letters DIN or Aph ID.
- 5.
- Do not remove the ABECMA infusion bag(s) from the cassette(s) if the information on the patient-specific cassette label(s) does not match the intended patient. Contact Bristol-Myers Squibb at 1-888-805-4555 if there are any discrepancies between the labels and the patient identifiers.
- 6.
- Once patient identity is confirmed, remove the ABECMA infusion bag(s) from the cassette(s) and check that the patient information on the cassette label(s) matches the patient information on the bag label(s).
- 7.
- Inspect the infusion bag(s) for any breaches of container integrity such as breaks or cracks before thawing. If the bag(s) is compromised, contact Bristol-Myers Squibb at 1-888-805-4555.
- 8.
- If more than one infusion bag has been received to achieve the treatment dose, thaw each infusion bag one at a time. Do not initiate thaw of the next bag until infusion of the previous bag is complete.
- 9.
- Place the infusion bag(s) inside a second sterile bag per local guidelines.
- 10.
- Thaw ABECMA infusion bag(s) at approximately 37°C using an approved thaw device or water bath until there is no visible ice in the infusion bag. Gently mix the contents of the bag to disperse clumps of cellular material. If visible cell clumps remain, continue to gently mix the contents of the bag. Small clumps of cellular material should disperse with gentle manual mixing. Do not wash, spin down, and/or resuspend ABECMA in new media prior to infusion.
- 11.
- ABECMA should be administered within 1 hour of the start of thaw. ABECMA is stable for 2 hours at room temperature once thawed.
ABECMA Administration
- •
- For autologous use only.
- •
- Do NOT use a leukodepleting filter.
- •
- Ensure that a minimum of 2 doses of tocilizumab and emergency equipment are available prior to infusion and during the recovery period.
- •
- Central venous access may be utilized for the infusion of ABECMA and is encouraged in patients with poor peripheral access.
- 1.
- Confirm that the patient's identity matches the patient identifiers on the ABECMA infusion bag(s).
- 2.
- Prime the tubing of the infusion set with normal saline prior to infusion.
- 3.
- Infuse the entire contents of the ABECMA infusion bag within 1 hour after start of thaw by gravity flow.
- 4.
- After the entire content of the infusion bag is infused, rinse the tubing with 30 to 60 mL of normal saline at the same infusion rate to ensure all product is delivered.
- 5.
- If more than one infusion bag has been received, administer all bags as directed, following steps 1-4 for all subsequent infusion bags. Do not initiate thaw of the next bag until infusion of the previous bag is complete.
ABECMA contains human blood cells that are genetically modified with replication-incompetent, self-inactivating lentiviral vector. Follow universal precautions and local biosafety guidelines for handling and disposal of ABECMA to avoid potential transmission of infectious diseases.
Monitoring
- •
- Administer ABECMA at a REMS-certified healthcare facility.
- •
- Monitor patients at least daily for 7 days following ABECMA infusion at the certified healthcare facility for signs and symptoms of CRS and neurologic toxicities [see Warnings and Precautions (5.1, 5.2)].
- •
- Instruct patients to remain within proximity of the certified healthcare facility for at least 4 weeks following infusion.
- •
- Instruct patients to refrain from driving or hazardous activities for at least 8 weeks following infusion.
2.3 Management of Severe Adverse Reactions
Cytokine Release Syndrome (CRS)
Identify CRS based on clinical presentation [see Warnings and Precautions (5.1)]. Evaluate for and treat other causes of fever, hypoxia, and hypotension.
If CRS is suspected, manage according to the recommendations in Table 1.
Patients who experience CRS should be closely monitored for cardiac and organ function until resolution of symptoms. Consider antiseizure prophylaxis with levetiracetam in patients who experience CRS.
Patients who experience Grade 2 or higher CRS (e.g., hypotension not responsive to fluids, or hypoxia requiring supplemental oxygenation) should be monitored with continuous cardiac telemetry and pulse oximetry.
For severe or life-threatening CRS, consider intensive care unit level monitoring and supportive therapy.
For CRS refractory to first line interventions such as tocilizumab or tocilizumab and corticosteroids, consider alternate treatment options (i.e., higher corticosteroid dose, alternative anti-cytokine agents, anti-T cell therapies). Refractory CRS is characterized by fevers, end-organ toxicity (e.g., hypoxia, hypotension) not improving within 12 hours of first line interventions or development of hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS).
If concurrent neurologic toxicity is suspected during CRS, administer:
- •
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 1 and 2
- •
- Tocilizumab according to the CRS grade in Table 1
- •
- Antiseizure medication according to the neurologic toxicity in Table 2
| CRS Gradea | Tocilizumabc | Corticosteroidsb |
|---|---|---|
| a Lee criteria for grading CRS (Lee et al., 2014). b If corticosteroids are initiated, continue corticosteroids for at least 3 doses, and taper over a maximum of 7 days. c Refer to tocilizumab Prescribing Information for details. |
||
|
Grade 1
|
If onset 72 hours or more after infusion, treat symptomatically. If onset less than 72 hours after infusion, consider tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg). |
Consider dexamethasone 10 mg IV every 24 hours. |
|
Grade 2
Oxygen requirement less than 40% FiO2 or hypotension responsive to fluids, or low dose of one vasopressor, or Grade 2 organ toxicity. |
Administer tocilizumab 8 mg/kg IV over 1 hour (not to exceed 800 mg). Limit to a maximum of 3 doses in a 24-hour period; maximum total of 4 doses. |
Consider dexamethasone 10 mg IV every 12-24 hours. |
|
If no improvement within 24 hours or rapid progression, repeat tocilizumab and escalate dose and frequency of dexamethasone (20 mg IV every 6 to 12 hours). If no improvement within 24 hours or continued rapid progression, switch to methylprednisolone 2 mg/kg followed by 2 mg/kg divided 4 times per day. After 2 doses of tocilizumab, consider alternative anti-cytokine agents. |
||
|
Grade 3
Fever, oxygen requirement greater than or equal to 40% FiO2, or hypotension requiring high-dose or multiple vasopressors, or Grade 3 organ toxicity or Grade 4 transaminitis. |
Per Grade 2 |
Administer dexamethasone 10 mg IV every 12 hours). |
|
If no improvement within 24 hours or rapid progression, repeat tocilizumab and escalate dose and frequency of dexamethasone (20 mg IV every 6 to 12 hours). After 2 doses of tocilizumab, consider alternative anti-cytokine agents. Do not exceed 3 doses of tocilizumab in 24 hours, or 4 doses in total. |
||
|
Grade 4
Requirements for ventilator support, continuous veno-venous hemodialysis (CVVHD), or Grade 4 organ toxicity (excluding transaminitis). |
Per Grade 2 |
Administer dexamethasone 20 mg IV every 6 hours. |
|
After 2 doses of tocilizumab, consider alternative anti-cytokine agents. Do not exceed 3 doses of tocilizumab in 24 hours, or 4 doses in total. If no improvement within 24 hours, consider methylprednisolone (1-2 g, repeat every 24 hours if needed; taper as clinically indicated) or other anti-T cell therapies. |
||
Neurologic Toxicity
Monitor patients for signs and symptoms of neurologic toxicities (Table 2). Rule out other causes of neurologic signs or symptoms. Provide intensive care supportive therapy for severe or life-threatening neurologic toxicities. If neurologic toxicity is suspected, manage according to the recommendations in Table 2.
If concurrent CRS is suspected during the neurologic toxicity event, administer:
- •
- Corticosteroids according to the more aggressive intervention based on the CRS and neurologic toxicity grades in Tables 1 and 2
- •
- Tocilizumab according to CRS grade in Table 1
- •
- Antiseizure medication according to neurologic toxicity in Table 2
| Neurologic Toxicity Gradea | Corticosteroids and Antiseizure Medications |
|---|---|
| a NCI CTCAE criteria for grading neurologic toxicities version 4.03. | |
|
Grade 1 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 2 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 3 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
|
Grade 4 |
Start non-sedating, antiseizure medicines (e.g., levetiracetam) for seizure prophylaxis. |
3. Dosage Forms and Strengths
ABECMA is a cell suspension for intravenous infusion.
A single dose of ABECMA contains a cell suspension of 300 to 460 × 106 chimeric antigen receptor (CAR)-positive T cells in one or more infusion bags [see How Supplied/Storage and Handling (16)].
5. Warnings and Precautions
5.1 Cytokine Release Syndrome (CRS)
CRS, including fatal or life-threatening reactions, occurred following treatment with ABECMA. CRS occurred in 85% (108/127) of patients receiving ABECMA. Grade 3 or higher CRS (Lee grading system1) occurred in 9% (12/127) of patients, with Grade 5 CRS reported in one (0.8%) patient. The median time-to-onset of CRS, any grade, was 1 day (range: 1 to 23 days), and the median duration of CRS was 7 days (range: 1 to 63 days) in all patients, including the patient who died. The most common manifestations of CRS included pyrexia (98%), hypotension (41%), tachycardia (35%), chills (31%), hypoxia (20%), fatigue (12%), and headache (10%). Grade 3 or higher events that may be associated with CRS include hypotension, hypoxia, hyperbilirubinemia, hypofibrinogenemia, ARDS, atrial fibrillation, hepatocellular injury, metabolic acidosis, pulmonary edema, multiple organ dysfunction syndrome and hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) [see Adverse Reactions (6.1)].
Identify CRS based on clinical presentation. Evaluate for and treat other causes of fever, hypoxia, and hypotension. CRS has been reported to be associated with findings of HLH/MAS, and the physiology of the syndromes may overlap. HLH/MAS is a potentially life-threatening condition. In patients with progressive symptoms of CRS or refractory CRS despite treatment, evaluate for evidence of HLH/MAS. Please see Section 5.3; Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome.
Overall rate of CRS was 79%, and rate of Grade 2 CRS was 23% in patients treated in the 300 × 106 CAR-positive T cells dose cohort (dose ranging from 277 to 339 × 106 CAR-positive T cells). For patients treated in the 450 × 106 CAR-positive T cells dose cohort (dose range 447 to 518 × 106 CAR-positive T cells), the overall rate of CRS was 96%, and rate of Grade 2 CRS was 40%. Rate of Grade 3 or higher CRS was similar across the dose range. The median duration of CRS for the 450 × 106 CAR-positive T cells dose cohort was 7 days (range 1 to 63 days), and was 6 days (range 2 to 28 days) for the 300 × 106 CAR-positive T cells dose cohort. In the 450 × 106 CAR-positive T cells dose cohort, 68% (36/53) of patients received tocilizumab and 23% (12/53) received at least 1 dose of corticosteroids for treatment of CRS. This was higher than the tocilizumab use of 44% (31/70) and corticosteroid use of 10% (7/70) at the 300 × 106 CAR-positive T cells dose cohort.
Sixty-eight of 127 (54%) patients received tocilizumab; 35% (45/127) received a single dose, while 18% (23/127) received more than 1 dose of tocilizumab. Overall, across the dose levels, 15% (19/127) of patients received at least 1 dose of corticosteroids for treatment of CRS. All patients that received corticosteroids for CRS also received tocilizumab.
Ensure that a minimum of 2 doses of tocilizumab are available prior to infusion of ABECMA.
Monitor patients at least daily for 7 days following ABECMA infusion at the REMS-certified healthcare facility for signs and symptoms of CRS. Monitor patients for signs or symptoms of CRS for at least 4 weeks after infusion. At the first sign of CRS, institute treatment with supportive care, tocilizumab and/or corticosteroids as indicated [see Dosage and Administration (2.3)].
Counsel patients to seek immediate medical attention should signs or symptoms of CRS occur at any time [see Patient Counseling Information (17)].
5.2 Neurologic Toxicities
Neurologic toxicities, which may be severe or life-threatening, occurred following treatment with ABECMA, including concurrently with CRS, after CRS resolution, or in the absence of CRS.
CAR T cell-associated neurotoxicity, occurred in 28% (36/127) of patients receiving ABECMA, including Grade 3 in 4% (5/127) of patients. One patient had ongoing Grade 2 neurotoxicity at the time of death. Two patients had ongoing Grade 1 tremor at the time of data cutoff. The median time to onset of neurotoxicity was 2 days (range: 1 to 42 days). CAR T cell-associated neurotoxicity resolved in 33 of 36 (92%). For patients who experienced neurotoxicity including three patients with ongoing neurotoxicity, the median duration of CAR T cell-associated neurotoxicity was 6 days (range: 1 to 578 days). Neurotoxicity resolved in 33 patients and median time to resolution was 5 days (range 1 to 61 days). Thirty-four patients with neurotoxicity had CRS. The onset of neurotoxicity during CRS was observed in 29 patients, before the onset of CRS in three patients, and after the CRS event in two patients.
The rate of Grade 3 neurotoxicity was 8% in 450 × 106 CAR-positive T cells dose cohort and 1.4% in the 300 × 106 CAR-positive T cells dose cohort. The most frequent (greater than or equal to 5%) manifestations of CAR T cell-associated neurotoxicity include encephalopathy (20%), tremor (9%), aphasia (7%), and delirium (6%).
Grade 4 neurotoxicity and cerebral edema have been associated with ABECMA in a patient in another study in multiple myeloma. Grade 3 myelitis and Grade 3 parkinsonism have occurred after treatment with ABECMA in another study in multiple myeloma.
Monitor patients at least daily for 7 days following ABECMA infusion at the REMS-certified healthcare facility for signs and symptoms of neurologic toxicities. Rule out other causes of neurologic symptoms. Monitor patients for signs or symptoms of neurologic toxicities for at least 4 weeks after infusion and treat promptly. Neurologic toxicity should be managed with supportive care and/or corticosteroids as needed [see Dosage and Administration (2.3)].
Counsel patients to seek immediate medical attention should signs or symptoms of neurologic toxicity occur at any time [see Patient Counseling Information (17)].
5.3 Hemophagocytic Lymphohistiocytosis (HLH)/ Macrophage Activation Syndrome (MAS)
HLH/MAS occurred in 4% (5/127) of patients receiving ABECMA. One patient treated in the 300 ×106 CAR-positive T cells dose cohort developed fatal multi-organ HLH/MAS with CRS. In another patient with fatal bronchopulmonary aspergillosis, HLH/MAS was contributory to the fatal outcome. Three cases of Grade 2 HLH/MAS resolved.
The rate of HLH/MAS was 8% in the 450 ×106 CAR-positive T cells dose cohort and 1% in the 300 ×106 CAR-positive T cells dose cohort. All events of HLH/MAS had onset within 10 days of receiving ABECMA, with a median onset of 7 days (range: 4 to 9 days) and occurred in the setting of ongoing or worsening CRS. Two patients with HLH/MAS had overlapping neurotoxicity.
The manifestations of HLH/MAS include hypotension, hypoxia, multiple organ dysfunction, renal dysfunction and cytopenia.
HLH/MAS is a potentially life-threatening condition with a high mortality rate if not recognized early and treated. Treatment of HLH/MAS should be administered per institutional standards.
5.4 ABECMA REMS
Because of the risk of CRS and neurologic toxicities, ABECMA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the ABECMA REMS [see Boxed Warning and Warnings and Precautions (5.1, 5.2)].
The required components of the ABECMA REMS are:
- •
- Healthcare facilities that dispense and administer ABECMA must be enrolled and comply with the REMS requirements.
- •
- Certified healthcare facilities must have on-site, immediate access to tocilizumab.
- •
- Ensure that a minimum of 2 doses of tocilizumab are available for each patient for infusion within 2 hours after ABECMA infusion, if needed for treatment of CRS.
- •
- Certified healthcare facilities must ensure that healthcare providers who prescribe, dispense, or administer ABECMA are trained in the management of CRS and neurologic toxicities.
- •
- Further information is available at www.AbecmaREMS.com or contact Bristol-Myers Squibb at 1-888-423-5436.
5.5 Hypersensitivity Reactions
Allergic reactions may occur with the infusion of ABECMA. Serious hypersensitivity reactions, including anaphylaxis, may be due to dimethyl sulfoxide (DMSO) in ABECMA.
5.6 Infections
ABECMA should not be administered to patients with active infections or inflammatory disorders. Severe, life-threatening, or fatal infections occurred in patients after ABECMA infusion. Infections (all grades) occurred in 70% of patients. Grade 3 or 4 infections occurred in 23% of patients. Grade 3 or 4 infections with an unspecified pathogen occurred in 15%, viral infections in 9%, bacterial infections in 3.9%, and fungal infections in 0.8% of patients. Overall, four patients had Grade 5 infections (3%); two patients (1.6%) had Grade 5 events of pneumonia, 1 patient (0.8%) had Grade 5 bronchopulmonary aspergillosis, and 1 patient (0.8%) had cytomegalovirus (CMV) pneumonia associated with Pneumocystis jirovecii. Monitor patients for signs and symptoms of infection before and after ABECMA infusion and treat appropriately. Administer prophylactic, pre-emptive, and/or therapeutic antimicrobials according to standard institutional guidelines.
Febrile neutropenia (was observed in 16% (20/127) of patients after ABECMA infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids, and other supportive care as medically indicated.
Viral Reactivation
Cytomegalovirus (CMV) infection resulting in pneumonia and death has occurred following ABECMA administration. Monitor and treat for CMV reactivation in accordance with clinical guidelines.
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, can occur in patients treated with drugs directed against plasma cells.
Perform screening for CMV, HBV, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) in accordance with clinical guidelines before collection of cells for manufacturing.
Consider antiviral therapy to prevent viral reactivation per local institutional guidelines/clinical practice.
5.7 Prolonged Cytopenias
Patients may exhibit prolonged cytopenias following lymphodepleting chemotherapy and ABECMA infusion. In the KarMMa study, 41% of patients (52/127) experienced prolonged Grade 3 or 4 neutropenia and 49% (62/127) experienced prolonged Grade 3 or 4 thrombocytopenia that had not resolved by Month 1 following ABECMA infusion. Rate of prolonged neutropenia was 49% in the 450 × 106 CAR-positive T cells dose cohort and 34% in the 300 × 106 CAR-positive T cells dose cohort. In 83% (43/52) of patients who recovered from Grade 3 or 4 neutropenia after Month 1, the median time to recovery from ABECMA infusion was 1.9 months. In 65% (40/62) of patients who recovered from Grade 3 or 4 thrombocytopenia, the median time to recovery was 2.1 months. Median time to cytopenia recovery was similar across the 300 and 450 × 106 CAR-positive T cells dose cohort.
Three patients underwent stem cell therapy (2 patients with autologous and 1 with allogeneic cells) for hematopoietic reconstitution due to prolonged cytopenia. Two of the three patients died from complications of prolonged cytopenia, which occurred in the setting of ongoing or prior severe CRS or HLH/MAS. Cause of death included lower gastrointestinal bleeding in the setting of prolonged thrombocytopenia in one patient and bronchopulmonary aspergillosis in the setting of prolonged neutropenia in another patient. The third patient recovered from neutropenia after autologous stem cell therapy.
Monitor blood counts prior to and after ABECMA infusion. Manage cytopenia with myeloid growth factor and blood product transfusion support according to local institutional guidelines.
5.8 Hypogammaglobulinemia
Plasma cell aplasia and hypogammaglobulinemia can occur in patients receiving treatment with ABECMA. Hypogammaglobulinemia was reported as an adverse event in 21% (27/127) of patients; laboratory IgG levels fell below 500 mg/dL after infusion in 25% (32/127) of patients treated with ABECMA.
Hypogammaglobulinemia either as an adverse reaction or laboratory IgG level below 500 mg/dL after infusion occurred in 41% (52/127) of patients treated with ABECMA. Sixty-one percent of patients received intravenous immunoglobulin (IVIG) post-ABECMA for serum IgG <400 mg/dL.
Monitor immunoglobulin levels after treatment with ABECMA and administer IVIG for IgG <400 mg/dL. Manage per local institutional guidelines, including infection precautions and antibiotic or antiviral prophylaxis.
Use of Live Vaccines
The safety of immunization with live viral vaccines during or following ABECMA treatment has not been studied. Vaccination with live virus vaccines is not recommended for at least 6 weeks prior to the start of lymphodepleting chemotherapy, during ABECMA treatment, and until immune recovery following treatment with ABECMA.
5.9 Secondary Malignancies
Patients treated with ABECMA may develop secondary malignancies. Monitor life-long for secondary malignancies. In the event that a secondary malignancy occurs, contact Bristol-Myers Squibb at 1-888-805-4555 for reporting and to obtain instructions on collection of patient samples for testing of secondary malignancy of T cell origin.
5.10 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including altered mental status or seizures, patients receiving ABECMA are at risk for altered or decreased consciousness or coordination in the 8 weeks following ABECMA infusion. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
6. Adverse Reactions/Side Effects
The following adverse reactions are described elsewhere in the labeling:
- •
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- •
- Neurologic Toxicities [see Warnings and Precautions (5.2)]
- •
- Hemophagocytic Lymphohistiocytosis (HLH)/Macrophage Activation Syndrome (MAS) [see Warnings and Precautions (5.3)]
- •
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- •
- Infections [see Warnings and Precautions (5.6)]
- •
- Prolonged Cytopenias [see Warnings and Precautions (5.7)]
- •
- Hypogammaglobulinemia [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety data described in this section reflect the exposure to ABECMA in the KarMMa study, in which 127 patients with relapsed/refractory multiple myeloma received ABECMA across a dose range of 150 to 518 × 106 CAR-positive T cells [see Clinical Studies (14)]. Patients with a history of CNS disease (such as seizure or cerebrovascular ischemia) or requiring ongoing treatment with chronic immunosuppression were excluded. The median duration of follow-up was 11.4 months. The median age of the study population was 61 years (range: 33 to 78 years); 35% were 65 years or older, and 60% were men. The Eastern Cooperative Oncology Group (ECOG) performance status at baseline was 0 in 45%, 1 in 53%, and 2 in 2% of patients. Seven percent of the patients treated with ABECMA had creatinine clearance <45 ml/min. For details about the study population, see Clinical Studies (14).
The most common (greater than or equal to 10%) Grade 3 or 4 nonlaboratory adverse reactions were febrile neutropenia (16%) and infections – pathogen unspecified (15%).
The most common nonlaboratory adverse reactions (incidence greater than or equal to 20%) included CRS, infections – pathogen unspecified, fatigue, musculoskeletal pain, hypogammaglobulinemia, diarrhea, upper respiratory tract infection, nausea, viral infections, encephalopathy, edema, pyrexia, cough, headache, and decreased appetite.
Serious adverse reactions occurred in 67% of patients. The most common nonlaboratory (greater than or equal to 5%) serious adverse reactions included CRS (18%), general physical health deterioration (10%), pneumonia (12%), infections-pathogen unspecified (19%), viral infections (9%), sepsis (7%), and febrile neutropenia (6%). Fatal adverse reactions occurred in 6%.
Table 3 summarizes the adverse reactions that occurred in at least 10% of patients treated with ABECMA. Table 4 describes the most common Grade 3 or 4 laboratory abnormalities.
| System Organ Class
Preferred Term | Target Dose of ABECMA (CAR-Positive T Cells) | |
|---|---|---|
| Any Grade | Grade 3 or Higher | |
| [150 to 450 × 106]
(N=127) % | [150 to 450 × 106]
(N=127) % |
|
| CAR=chimeric antigen receptor. a Tachycardia includes sinus tachycardia, tachycardia. b Oral pain includes oral pain, oropharyngeal pain, toothache. c Fatigue includes asthenia, fatigue, malaise. d Edema includes edema, face edema, fluid overload, fluid retention, generalized edema, peripheral edema, peripheral swelling, scrotal swelling, swelling. e Hypogammaglobulinemia includes patients with adverse events (21%) of blood immunoglobulin G decreased, hypogammaglobulinemia, hypoglobulinemia; and/or patients with laboratory IgG levels below 500 mg/dL following ABECMA infusion (25%). f Infections and infestations System Organ Class Adverse Events are grouped by pathogen type and selected clinical syndromes. g Pneumonia includes bronchopulmonary aspergillosis, lung infection, pneumonia, pneumonia aspiration, pneumonia cytomegaloviral, pneumonia pneumococcal, pneumonia pseudomonal. Pneumonias may also be included under pathogen categories. h Upper respiratory tract infection includes laryngitis, nasopharyngitis, pharyngeal erythema, pharyngitis, respiratory tract congestion, respiratory tract infection, rhinitis, rhinovirus infection, sinusitis, upper respiratory tract infection, upper respiratory tract infection bacterial. Upper respiratory tract infections may also be included under pathogen categories. i Decreased appetite includes decreased appetite, hypophagia. j Musculoskeletal pain includes arthralgia, back pain, bone pain, musculoskeletal chest pain, musculoskeletal discomfort, musculoskeletal pain, musculoskeletal stiffness, myalgia, neck pain, spinal pain. k Motor dysfunction includes dysphonia, eyelid ptosis, hypotonia, motor dysfunction, muscle spasms, muscular weakness, restless legs syndrome. l Encephalopathy includes amnesia, bradyphrenia, cognitive disorder, confusional state, depressed level of consciousness, disturbance in attention, dyscalculia, dysgraphia, encephalopathy, lethargy, memory impairment, mental status changes, metabolic encephalopathy, somnolence, toxic encephalopathy. m Headache includes headache, head discomfort, sinus headache. n Dizziness includes dizziness, presyncope, syncope, vertigo. o Neuropathy peripheral includes carpal tunnel syndrome, hypoesthesia, hypoesthesia oral, neuralgia, neuropathy peripheral, paresthesia, peripheral sensorimotor neuropathy, peripheral sensory neuropathy, sciatica. p Tremor includes asterixis, tremor. q Insomnia includes insomnia, sleep deficit, sleep disorder. r Anxiety includes anxiety, feeling jittery, nervousness. s Renal failure includes acute kidney injury, blood creatinine increased, chronic kidney disease, renal failure, renal impairment. t Cough includes cough, productive cough, upper-airway cough syndrome. u Dyspnea includes acute respiratory failure, dyspnea, dyspnea exertional, respiratory failure. v Rash includes acne, dermatitis, dermatitis bullous, erythema, rash, rash macular, rash papular, urticaria. w Xerosis includes dry eye, dry mouth, dry skin, lip dry, xerosis. x Hypotension includes hypotension, orthostatic hypotension. |
||
|
Blood and lymphatic system disorders | ||
|
Febrile neutropenia |
16 |
16 |
|
Cardiac disorders | ||
|
Tachycardiaa |
19 |
0 |
|
Gastrointestinal disorders | ||
|
Diarrhea |
35 |
1.6 |
|
Nausea |
29 |
0 |
|
Constipation |
16 |
0 |
|
Vomiting |
15 |
0 |
|
Oral painb |
12 |
0 |
|
General disorders and administration site conditions | ||
|
Fatiguec |
45 |
3.1 |
|
Pyrexia |
25 |
1.6 |
|
General physical health deterioration |
11 |
10 |
|
Edemad |
25 |
0 |
|
Chills |
11 |
0 |
|
Immune system disorders | ||
|
Cytokine release syndrome |
85 |
9 |
|
Hypogammaglobulinemiae |
41 |
0.8 |
|
Infections and infestationsf | ||
|
Infections – Pathogen unspecified |
51 |
15 |
|
Viral infections |
27 |
9 |
|
Bacterial infections |
15 |
3.9 |
|
Pneumoniag |
17 |
9 |
|
Upper respiratory tract infectionh |
34 |
1.6 |
|
Investigations | ||
|
Weight decreased |
13 |
1.6 |
|
Metabolism and nutrition disorders | ||
|
Decreased appetitei |
22 |
0.8 |
|
Musculoskeletal and connective tissue disorders | ||
|
Musculoskeletal painj |
45 |
3.1 |
|
Motor dysfunctionk |
11 |
0 |
|
Nervous system disorders | ||
|
Encephalopathyl |
26 |
6 |
|
Headachem |
23 |
0 |
|
Dizzinessn |
17 |
0.8 |
|
Neuropathy peripheralo |
17 |
0.8 |
|
Tremorp |
10 |
0 |
|
Psychiatric disorders | ||
|
Insomniaq |
13 |
0 |
|
Anxietyr |
12 |
0.8 |
|
Renal and urinary disorders | ||
|
Renal failures |
10 |
2.4 |
|
Respiratory, thoracic, and mediastinal disorders | ||
|
Cought |
23 |
0 |
|
Dyspneau |
13 |
2.4 |
|
Skin and subcutaneous tissue disorder | ||
|
Rashv |
14 |
0.8 |
|
Xerosisw |
11 |
0 |
|
Vascular disorders | ||
|
Hypotensionx |
17 |
0 |
|
Hypertension |
11 |
3.1 |
Other clinically important adverse reactions that occurred in less than 10% of patients treated with ABECMA include the following:
- •
- Blood and lymphatic system disorders: coagulopathya (9%)
- •
- Cardiac disorders: atrial fibrillation (4.7%), cardiomyopathyb (1.6%)
- •
- Gastrointestinal disorders: gastrointestinal hemorrhagec (3.1%)
- •
- Immune system disorders: hemophagocytic lymphohistiocytosis (3.1%)
- •
- Infections and infestations: fungal infections (8%), sepsisd (9%)
- •
- Nervous system disorders: aphasiae (7%), ataxiaf (3.1%), paresisg (2.4%), seizure (1.6%)
- •
- Psychiatric disorders: deliriumh (6%)
- •
- Respiratory, thoracic, and mediastinal disorders: hypoxia (2.4%), pulmonary edema (2.4%)
- •
- Vascular disorders: thrombosisi (3.1%)
a Coagulopathy includes activated partial thromboplastin time prolonged, anticoagulation drug level above therapeutic, disseminated intravascular coagulation, international normalized ratio increased.
b Cardiomyopathy includes stress cardiomyopathy, ventricular hypertrophy.
c Gastrointestinal hemorrhage includes gastrointestinal hemorrhage, hemorrhoidal hemorrhage, melena.
d Sepsis includes bacteremia, enterococcal bacteremia, Escherichia bacteremia, sepsis, septic shock, Serratia bacteremia, streptococcal bacteremia.
e Aphasia includes aphasia, dysarthria.
f Ataxia includes ataxia, gait disturbance, Romberg test positive.
g Paresis includes cranial nerve disorder, hemiparesis.
h Delirium includes delirium, disorientation, hallucination.
i Thrombosis includes deep vein thrombosis, jugular vein thrombosis, portal vein thrombosis, pulmonary embolism.
Laboratory Abnormalities
Table 4 presents the most common Grade 3 or 4 laboratory abnormalities, based on laboratory data, occurring in at least 10% of patients.
| Laboratory Abnormality | Dose=[150 to 450 × 106 CAR-Positive T cells]
(N=127) % |
|---|---|
| aNCI CTCAE=Common Terminology Criteria for Adverse Events version 4.03. aPTT=activated partial thromboplastin time; CAR=chimeric antigen receptor; CTCAE=Common Terminology Criteria for Adverse Events; NCI=National Cancer Institute. Laboratory tests were graded according to NCI CTCAE Version 4.03. Laboratory abnormalities are sorted by decreasing frequency in the 150 to 450 × 106 column. |
|
|
Grade 3 or 4 (%) |
|
|
Neutropenia |
96 |
|
Leukopenia |
96 |
|
Lymphopenia |
92 |
|
Thrombocytopenia |
63 |
|
Anemia |
63 |
|
Hypophosphatemia |
45 |
|
Hyponatremia |
10 |
|
aPTT Increased (seconds) |
10 |
Other clinically important Grade 3 or 4 laboratory abnormalities (based on laboratory data) that occurred in less than 10% of patients treated with ABECMA include the following: alanine aminotransferase increased, aspartate aminotransferase increased, hypoalbuminemia, alkaline phosphatase increased, hyperglycemia, hypokalemia, bilirubin increased, hypofibrinogenemia, and hypocalcemia.
6.2 Immunogenicity
ABECMA has the potential to induce anti-product antibodies. In clinical studies, humoral immunogenicity of ABECMA was measured by determination of anti-CAR antibody in serum pre- and post-administration. In the KarMMa study, 3% of patients (4/127) tested positive for pre-infusion anti-CAR antibodies and treatment-induced anti-CAR antibodies were detected in 47% (60/127) of the patients. There is no evidence that the presence of pre-existing or post-infusion anti-CAR antibodies impact the cellular expansion, safety, or effectiveness of ABECMA.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data with ABECMA use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with ABECMA to assess whether it can cause fetal harm when administered to a pregnant woman.
It is not known if ABECMA has the potential to be transferred to the fetus. Based on the mechanism of action, if the transduced cells cross the placenta, they may cause fetal toxicity, including plasma cell aplasia or hypogammaglobulinemia. Therefore, ABECMA is not recommended for women who are pregnant, and pregnancy after ABECMA infusion should be discussed with the treating physician. Assess immunoglobulin levels in newborns of mothers treated with ABECMA.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. The estimated background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
8.2 Lactation
Risk Summary
There is no information regarding the presence of ABECMA in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ABECMA and any potential adverse effects on the breastfed infant from ABECMA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Pregnancy status of sexually-active females with reproductive potential should be verified via pregnancy testing prior to starting treatment with ABECMA.
Contraception
See the prescribing information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive the lymphodepleting chemotherapy.
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with ABECMA.
8.4 Pediatric Use
The safety and efficacy of ABECMA in patients under 18 years of age have not been established.
8.5 Geriatric Use
In the clinical trial of ABECMA, 45 (35%) of the 127 patients in the KarMMa study were 65 years of age or older and 4/127 (3%) patients were 75 years of age or older. All five cases of Grade 3 neurotoxicity occurred in patients ≥65 years of age (66 to 74 years). No clinically important differences in effectiveness of ABECMA were observed between these patients and patients younger than 65 years of age.
11. Abecma Description
ABECMA is a BCMA-directed genetically modified autologous T cell immunotherapy product consisting of a patient's own T cells that are harvested and genetically modified ex vivo through transduction with an anti-BCMA02 chimeric antigen receptor (CAR) lentiviral vector (LVV). Autologous T cells transduced with the anti-BCMA02 CAR LVV express the anti-BCMA CAR on the T cell surface. The CAR is comprised of a murine extracellular single-chain variable fragment (scFv) specific for recognizing B cell maturation antigen (BCMA) followed by a human CD8α hinge and transmembrane domain fused to the T cell cytoplasmic signaling domains of CD137 (4-1BB) and CD3ζ chain, in tandem. Binding of ABECMA to BCMA-expressing target cells leads to signaling initiated by CD3ζ and 4-1BB domains, and subsequent CAR-positive T cell activation. Antigen-specific activation of ABECMA results in CAR-positive T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells.
ABECMA is prepared from the patient's peripheral blood mononuclear cells (PBMCs), which are obtained via a standard leukapheresis procedure. The mononuclear cells are enriched for T cells, through activation with anti-CD3 and anti-CD28 antibodies in the presence of IL-2, which are then transduced with the replication-incompetent lentiviral vector containing the anti-BCMA CAR transgene. The transduced T cells are expanded in cell culture, washed, formulated into a suspension, and cryopreserved. The product must pass a sterility test before release for shipping as a frozen suspension in one or more patient-specific infusion bag(s). The product is thawed prior to infusion back into the patient [see Dosage and Administration (2.3) and How Supplied/Storage and Handling (16)].
The ABECMA formulation contains 50% Plasma-Lyte A and 50% CryoStor® CS10, resulting in a final DMSO concentration of 5%.
12. Abecma - Clinical Pharmacology
12.1 Mechanism of Action
ABECMA is a chimeric antigen receptor (CAR)-positive T cell therapy targeting B-cell maturation antigen (BCMA), which is expressed on the surface of normal and malignant plasma cells. The CAR construct includes an anti-BCMA scFv-targeting domain for antigen specificity, a transmembrane domain, a CD3-zeta T cell activation domain, and a 4-1BB costimulatory domain. Antigen-specific activation of ABECMA results in CAR-positive T cell proliferation, cytokine secretion, and subsequent cytolytic killing of BCMA-expressing cells.
12.2 Pharmacodynamics
Following ABECMA infusion, pharmacodynamic responses of CAR activation and anti-tumor efficacy were evaluated. Peak elevation of plasma cytokines, chemokines, and soluble immune mediators occurred within 14 days of ABECMA infusion and returned to baseline levels within one month.
Rapid decreases in tumor markers associated with clinical response, including serum levels of soluble BCMA, and bone marrow CD138+ cells, as well as minimal residual disease (MRD) negative responses, were observed within the first month following ABECMA infusion.
12.3 Pharmacokinetics
Following ABECMA infusion, the CAR-positive cells proliferate and undergo rapid multi-log expansion followed by a bi-exponential decline. The median time of maximal expansion in peripheral blood (Tmax) occurred 11 days after infusion.
ABECMA can persist in peripheral blood for up to 1 year post-infusion. A summary of Tmax, AUC0-28days, and Cmax by the recommended dose range provided in Table 5.
| Pharmacokinetic Parameter | Summary Statistic | Total
[300 to 460 × 106] CAR-Positive T Cells |
|---|---|---|
| AUC0-28days = area under the curve of the transgene level from time of dose to 28 days post-infusion; Cmax = the maximum transgene level; Tmax = time of maximum observed transgene level. | ||
|
Tmax (days) |
Median (Range) |
11 (7-28) |
|
Cmax (copies/mcg) |
Geometric mean (geometric CV%) |
256,333 (165) |
|
AUC0-28days (days*copies/mcg) |
Geometric mean (geometric CV%) |
3,088,455 (190) |
ABECMA transgene levels were positively associated with objective tumor response (partial response or better). The median Cmax levels in responders (N = 72) were approximately 4.6-fold higher than the corresponding levels in non-responders (N = 27). Median AUC0-28days in responding patients (N = 72) was approximately 5.6-fold higher than non-responders (N = 26).
Tocilizumab and Corticosteroid Use
Some patients required tocilizumab and/or corticosteroid for the management of CRS. ABECMA can continue to expand and persist following tocilizumab or corticosteroid administration [see Warnings and Precautions (5.1)].
Patients with CRS treated with tocilizumab had higher ABECMA cellular expansion levels, as measured by 1.3-fold and 1.6-fold higher median Cmax (N = 67) and AUC0-28days (N = 66), respectively, compared to patients who did not receive tocilizumab (N = 59 for Cmax and N = 58 for AUC0-28days).
Patients with CRS treated with corticosteroids had higher ABECMA cellular expansion levels, as measured by 1.7-fold and 2.2-fold higher median Cmax (N = 18) and AUC0-28days (N = 18), respectively, compared to patients who did not receive corticosteroids (N = 108 for Cmax and N = 106 for AUC0-28days).
Specific Populations
Geriatric
Age (range: 33 to 78 years) had no significant impact on expansion parameters [see Use in Special Populations (8.5)].
Pediatric
The pharmacokinetics of ABECMA in patients less than 18 years of age have not been evaluated.
Patients with Hepatic/Renal Impairment
Hepatic and renal impairment studies of ABECMA were not conducted.
Patients with Other Intrinsic Factors
Gender, race, and ethnicity had no significant impact on ABECMA expansion parameters. Patients with lower body weight had higher expansion. Due to high variability in pharmacokinetic cellular expansion, the overall effect of weight on the pharmacokinetics of ABECMA is considered to be not clinically relevant.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Genotoxicity assays and carcinogenicity studies in rodents were not performed for ABECMA
In vitro expansion studies with CAR-positive T cells (ABECMA) from 5 patients and 2 healthy donor drug product lots showed no evidence for transformation and/or immortalization of T cells. A genomic insertion site analysis of the lentiviral vector was performed on ABECMA samples from twenty (20) individual patient donors. There was no evidence for preferential integration near genes of concern or preferential outgrowth of cells harboring integration sites of concern.
No studies on the effects of ABECMA on fertility have been conducted.
14. Clinical Studies
Relapsed/Refractory Multiple Myeloma
Efficacy of ABECMA was evaluated in KarMMa (NCT03361748), an open-label, single-arm, multicenter study in adult patients with relapsed and refractory multiple myeloma who had received at least 3 prior lines of antimyeloma therapy including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. The study included patients with ECOG performance status of 0 or 1. The study excluded patients with a creatinine clearance of less than or equal to 45 mL/minute, alanine aminotransferase >2.5 times upper limit of normal and left ventricular ejection fraction <45%. Patients were also excluded if absolute neutrophil count <1000 cells/mm3 and platelet count <50,000/mm3. Patients had measurable disease by International Myeloma Working Group (IMWG) 2016 criteria at enrollment. Bridging therapy with alkylating agents, corticosteroids, immunomodulatory agents, proteasome inhibitors, and/or anti-CD38 monoclonal antibodies to which patients were previously exposed was permitted for disease control between apheresis and until 14 days before the start of lymphodepleting chemotherapy.
Lymphodepleting chemotherapy consisted of cyclophosphamide (300 mg/m2 IV infusion daily for 3 days) and fludarabine (30 mg/m2 IV infusion daily for 3 days) starting 5 days prior to the target infusion date of ABECMA. Fludarabine was dose reduced for renal insufficiency. Patients were hospitalized for 14 days after ABECMA infusion to monitor for potential CRS, HLH/MAS, and neurotoxicity.
Of the 135 patients who underwent leukapheresis for 300 × 106 and 450 × 106 CAR-positive T cell dose cohorts:
- •
- 11 (8%) did not receive the CAR-positive T cells either due to death (n=2), adverse event (n=1), disease progression (n=1), consent withdrawal (n=3), physician decision (n=3), or inability to manufacture product [manufacturing failure (n=1)]. Two patients died after receiving lymphodepletion and prior to receiving ABECMA. Deaths were from septic shock and general physical health deterioration.
- •
- 24 (18%) either received ABECMA outside of the 300 to 460 × 106 CAR-positive T cells dose range (n=23) or received CAR-positive T cells that did not meet product release specifications for ABECMA (non-conforming product; n=1).
- •
- The efficacy evaluable population consists of the 100 patients (74%) who received ABECMA in the dose range of 300 to 460 × 106 CAR-positive T cells.
The overall manufacturing failure rate for patients who underwent leukapheresis for the 300 × 106 and 450 × 106 CAR-positive T cell dose cohorts was 1.5% (2 out of 135 patients). Of these 2 patients, one received CAR-positive T cells that did not meet product release specifications for ABECMA, and in one patient there was an inability to manufacture ABECMA.
Of the 100 patients in the efficacy evaluable population, the median age was 62 years (range: 33 to 78 years), 60% were male, 78% were white, 6% were black, and 2% were Asian. Most patients (78%) were International Staging System (ISS) Stage I or II. High-risk cytogenetics (presence of t(4:14), t(14:16), and 17p13 del) were present in 37% of patients. Thirty-six percent of the patients had presence of extramedullary disease.
The median number of prior lines of therapy was 6 (range: 3 to 16), and 88% of the patients received 4 or more prior lines of therapy. Ninety-five percent of the patients were refractory to an anti-CD38 monoclonal antibody. Eighty-five percent were triple class refractory (refractory to a proteasome inhibitor [PI], an immunomodulatory drug [IMiD] and an anti-CD38 monoclonal antibody), and 26% were penta-refractory (refractory to 2 PIs, 2 IMiD agents, and an anti-CD38 monoclonal antibody). Ninety-two percent had received prior autologous stem cell transplantation.
Most patients (87%) treated with ABECMA received bridging therapy for control of their multiple myeloma during the manufacturing process. The median time from leukapheresis to product availability was 33 days (range: 26 to 49 days).
Efficacy was established on the basis of overall response rate (ORR), complete response (CR) rate, and duration of response (DOR), as assessed by the Independent Response committee (IRC) based on the International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma.
Efficacy results for the dose range of 300 to 460 × 106 CAR-positive T cells are shown in Table 6 and Table 7, and the DOR results are shown in Table 8. The median time to first response was 30 days (range: 15 to 88 days).
| ABECMA-Treated Population (300 to 460 × 106 CAR-Positive T Cells)
N=100 |
|
|---|---|
| CAR=chimeric antigen receptor; CI=confidence interval; CR=complete response; MRD=Minimal Residual Disease; IMWG=International Myeloma Working Group; PR=partial response; sCR=stringent complete response; VGPR=very good partial response. a All complete responses were stringent CRs. b: Clopper-Pearson exact CI. |
|
|
Overall Response Rate (sCRa+VGPR+PR), n (%) |
72 (72) |
|
sCRa, n (%) |
28 (28) |
|
VGPR, n (%) |
25 (25) |
|
PR, n (%) |
19 (19) |
| a MRD negativity was defined as the proportion of patients with CR or stringent CR who are MRD negative at any timepoint within 3 months prior to achieving CR or stringent CR until the time of progression or death. b Clopper-Pearson exact CI. c Based on a threshold of 10-5 using ClonoSEQ, a next-generation sequencing assay (NGS). |
|||
|
MRDc-negativity ratea in all treated patients (n=100) |
21 (21) |
||
|
95% CIb (%) |
13, 30 |
||
|
MRDc-negativity ratea in patients achieving CR or sCR status (%) (n=28) |
21 (75) |
||
|
95% CIb |
55, 89 |
||
| ABECMA-Treated Population (300 to 460 × 106 CAR-Positive T Cells)
N=100 |
|||||
|---|---|---|---|---|---|
| CAR=chimeric antigen receptor; CI=confidence interval; CR=complete response; PR=partial response; sCR=stringent complete response; VGPR=very good partial response; NE=not estimable. a Response is defined as achieving sCR, CR, VGPR, or PR according to IMWG criteria. b Median and 95% CI are based on Kaplan-Meier estimation. |
|||||
|
Duration of Responsea,b (PR or Better) | |||||
|
n |
72 |
||||
|
Median (months) |
11.0 |
||||
|
95% CI |
10.3, 11.4 |
||||
|
Duration of Responseb for sCR | |||||
|
n |
28 |
||||
|
Median (months) |
19.0 |
||||
|
95% CI |
11.4, NE |
||||
|
Median follow-up for duration of response (DOR) |
10.7 months |
||||
Response durations were longer in patients who achieved a stringent CR as compared to patients with a PR or VGPR (Table 8). Of the 28 patients who achieved a stringent CR, it is estimated that 65% (95% CI: 42%, 81%) had a remission lasting at least 12 months.
The median duration of response for VGPR patients (n=25) was 11.1 months (95% CI: 8.7, 11.3).
The median duration of response for PR patients (n=19) was 4.0 months (95% CI: 2.7, 7.2).
Within the recommended dose of 300 to 460 × 106 CAR-positive T cells, a dose-response relationship was observed with higher ORR and sCR rate in patients who received 440 to 460 × 106 compared to 300 to 340 × 106 CAR-positive T cells. Overall response rate of 79% (95% CI: 65%, 90%) and sCR rate of 31% (95% CI: 19%, 46%) was observed with 440 to 460 × 106 CAR-positive T cells. Overall response rate of 65% (95% CI: 51%, 78%) with sCR rate of 25% (95% CI: 14%, 39%) was observed in 300 to 340 × 106 CAR-positive T cells.
One hundred and thirty-five patients underwent leukapheresis. Fifteen out of the 23 patients who received treatment outside of the recommended dose range of 300 to 460 × 106 CAR-positive T cells experienced a response in addition to the responses noted in Table 6. The IRC assessed overall response in the leukapheresis population (n=135) was 64% (95% CI: 56%, 72%) with stringent CR rate of 24% (95% CI: 17%, 32%), VGPR rate of 21% (95% CI: 14%, 29%) and PR rate of 20% (95% CI: 14%, 28%).
15. References
- 1.
- Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124(2): 188-95. Errata in Blood: 2015;126(8):1048. and 2016;128(11):1533.
- 2.
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016; 17(8): e328-46.
16. How is Abecma supplied
ABECMA is supplied in one or more infusion bag(s) (see below) containing a frozen suspension of genetically modified autologous T cells in 5% DMSO.
Each infusion bag of ABECMA is individually packed in a metal cassette. ABECMA is stored in the vapor phase of liquid nitrogen and supplied in a liquid nitrogen dry vapor shipper. An RFI Certificate is affixed inside the shipper.
- •
- 50 mL infusion bag and metal cassette (NDC 59572-515-01)
- •
- 250 mL infusion bag and metal cassette (NDC 59572-515-02)
- •
- 500 mL infusion bag and metal cassette (NDC 59572-515-03)
Match the identity of the patient with the patient identifiers on the cassette(s) and infusion bag(s) upon receipt.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Ensure that patients understand the risk of manufacturing failure (1.5%, [2/135 in the clinical study]). In case of a manufacturing failure, a second manufacturing of ABECMA may be attempted. In addition, while the patient awaits the product, additional anticancer treatment (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period, which could delay or prevent the administration of ABECMA.
Advise patients to seek immediate attention for any of the following:
- •
- Cytokine Release Syndrome (CRS): Signs or symptoms associated with CRS, including fever, hypotension, tachycardia, chills, hypoxia, headache, and fatigue [see Dosage and Administration (2.3), Warnings and Precautions (5.1), and Adverse Reactions (6.1)].
- •
- Neurologic Toxicities: Signs or symptoms associated with neurologic events, including encephalopathy, confusion, seizures, tremor, aphasia, delirium, and somnolence [see Dosage and Administration (2.3), Warnings and Precautions (5.2), and Adverse Reactions (6.1)].
- •
- Infections: Signs or symptoms associated with infection [see Warnings and Precautions (5.6) and Adverse Reactions (6.1)].
- •
- Prolonged Cytopenias: Signs or symptoms associated with bone marrow suppression, including neutropenia, anemia, thrombocytopenia, or febrile neutropenia [see Warnings and Precautions (5.7) and Adverse Reactions (6.1)].
Advise patients for the need to:
- •
- Contact Bristol-Myers Squibb at 1-888-805-4555 if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.9)].
- •
- Have periodic monitoring of blood counts before and after ABECMA infusion [see Warnings and Precautions (5.6)].
- •
- Refrain from driving or operating heavy or potentially dangerous machines until at least 8 weeks after ABECMA administration [see Warnings and Precautions (5.10)].
Manufactured by: Celgene Corporation, a Bristol-Myers Squibb Company
556 Morris Avenue
Summit, NJ 07901
U.S License No. 2252
Marketed by:
Celgene Corporation, a Bristol-Myers Squibb Company (Summit, NJ 07901), and bluebird bio, Inc. (Cambridge, MA 02142)
ABECMA® is a registered trademark of Celgene Corporation, a Bristol-Myers Squibb Company.
© 2021 Celgene Corporation, a Bristol-Myers Squibb Company. All Rights Reserved.
ABEPI.001/MG.001
| MEDICATION GUIDE
ABECMA® (uh-BEK-muh) (idecabtagene vicleucel) |
|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: MAR 2021 |
|
Read this Medication Guide before you start your ABECMA treatment. The more you know about your treatment, the more active you can be in your care. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. |
|
What is the most important information I should know about ABECMA?
It is important that you tell your healthcare providers that you have received ABECMA and to show them your ABECMA Patient Wallet Card. Your healthcare provider may give you other medicines to treat your side effects. |
|
What is ABECMA?
|
|
How will I receive ABECMA?
|
|
What should I avoid after receiving ABECMA?
|
|
What are the possible or reasonably likely side effects of ABECMA?
ABECMA can cause a very common side effect called cytokine release syndrome or CRS, which can be severe or fatal. Symptoms of CRS include fever, difficulty breathing, dizziness or light-headedness, nausea, headache, fast heartbeat, low blood pressure, or fatigue. Tell your healthcare provider right away if you develop fever or any of these other symptoms after receiving ABECMA. |
|
General information about the safe and effective use of ABECMA
|
PRINCIPAL DISPLAY PANEL - 30 mL Bag Label
idecabtagene vicleucel
Abecma™
NDC 59572-515-01
Genetically modified autologous T cells
FOR AUTOLOGOUS AND INTRAVENOUS USE ONLY
Suspension for IV Infusion
Rx Only
Acceptable Volume 10mL - 30mL per bag
Dosage: See prescribing information and
Release for Infusion Certificate (inside shipper).
Contains: 300 to 460 x 106 anti-BCMA02
CAR-positive T cells in cryopreservative
solution containing 5% DMSO USP.
Dose may be suspended in 1 or more
infusion bag(s).
Do not use a leukodepleting filter
or irradiate.
Not evaluated for infectious substances.
Preservative free.
Store in vapor phase of liquid nitrogen
(≤ -130°C).
Mfd by: Celgene Corporation,
a Bristol-Myers Squibb Company
Summit, NJ 07901 USA
Phone: 1-888-805-4555
US License No. 2252
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
DIN/Aph ID: W0000 00 000000
JOIN: XXXX-XXXXX
LOT: XXXX-XXXXXY
EXP: DD-MMM-YYYY
Bag ID: XX
XXXX-XXXXXY-XX
303400
PRINCIPAL DISPLAY PANEL - 30 mL Cassette Label
idecabtagene vicleucel
Abecma™
NDC 59572-515-01
Genetically modified autologous T cells
FOR AUTOLOGOUS AND INTRAVENOUS USE ONLY
Suspension for IV Infusion
Rx Only
Acceptable Volume 10mL - 30mL per bag
Dosage: See prescribing information and Release for
Infusion Certificate (inside shipper).
Contains: 300 to 460 x 106 anti-BCMA02 CAR-positive T cells
in cryopreservative solution containing 5% DMSO USP.
Dose may be suspended in 1 or more infusion bag(s).
Do not use a leukodepleting filter or irradiate.
Not evaluated for infectious substances. Preservative free.
Store in vapor phase of liquid nitrogen (≤ -130°C).
Mfd by: Celgene Corporation, a Bristol-Myers Squibb Company
Summit, NJ 07901 USA
Phone: 1-888-805-4555
US License No. 2252
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
DIN/Aph ID: W0000 00 000000
JOIN: XXXX-XXXXX
LOT: XXXX-XXXXXY
EXP: DD-MMM-YYYY
Bag ID: XX
XXXX-XXXXXY-XX
303402
PRINCIPAL DISPLAY PANEL - 70 mL Bag Label
idecabtagene vicleucel
Abecma™
NDC 59572-515-02
Genetically modified autologous T cells
FOR AUTOLOGOUS AND INTRAVENOUS USE ONLY
Suspension for IV Infusion
Rx Only
Acceptable Volume 30mL - 70mL per bag
Dosage: See prescribing information and
Release for Infusion Certificate (inside shipper).
Contains: 300 to 460 x 106 anti-BCMA02
CAR-positive T cells in cryopreservative
solution containing 5% DMSO USP.
Dose may be suspended in 1 or more
infusion bag(s).
Do not use a leukodepleting filter
or irradiate.
Not evaluated for infectious substances.
Preservative free.
Store in vapor phase of liquid nitrogen
(≤ -130°C).
Mfd by: Celgene Corporation,
a Bristol-Myers Squibb Company
Summit, NJ 07901 USA
Phone: 1-888-805-4555
US License No. 2252
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
DIN/Aph ID: W0000 00 000000
JOIN: XXXX-XXXXX
LOT: XXXX-XXXXXY
EXP: DD-MMM-YYYY
Bag ID: XX
XXXX-XXXXXY-XX
303400
PRINCIPAL DISPLAY PANEL - 70 mL Cassette Label
idecabtagene vicleucel
Abecma™
NDC 59572-515-02
Genetically modified autologous T cells
FOR AUTOLOGOUS AND INTRAVENOUS USE ONLY
Suspension for IV Infusion
Rx Only
Acceptable Volume 30mL - 70mL per bag
Dosage: See prescribing information and Release for
Infusion Certificate (inside shipper).
Contains: 300 to 460 x 106 anti-BCMA02 CAR-positive T cells
in cryopreservative solution containing 5% DMSO USP.
Dose may be suspended in 1 or more infusion bag(s).
Do not use a leukodepleting filter or irradiate.
Not evaluated for infectious substances. Preservative free.
Store in vapor phase of liquid nitrogen (≤ -130°C).
Mfd by: Celgene Corporation, a Bristol-Myers Squibb Company
Summit, NJ 07901 USA
Phone: 1-888-805-4555
US License No. 2252
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
DIN/Aph ID: W0000 00 000000
JOIN: XXXX-XXXXX
LOT: XXXX-XXXXXY
EXP: DD-MMM-YYYY
Bag ID: XX
XXXX-XXXXXY-XX
303402
PRINCIPAL DISPLAY PANEL - 100 mL Bag Label
idecabtagene vicleucel
Abecma™
NDC 59572-515-03
Genetically modified autologous T cells
FOR AUTOLOGOUS AND INTRAVENOUS USE ONLY
Suspension for IV Infusion
Rx Only
Acceptable Volume 55mL - 100mL per bag
Dosage: See prescribing information and
Release for Infusion Certificate (inside shipper).
Contains: 300 to 460 x 106 anti-BCMA02
CAR-positive T cells in cryopreservative
solution containing 5% DMSO USP.
Dose may be suspended in 1 or more
infusion bag(s).
Do not use a leukodepleting filter
or irradiate.
Not evaluated for infectious substances.
Preservative free.
Store in vapor phase of liquid nitrogen
(≤ -130°C).
Mfd by: Celgene Corporation,
a Bristol-Myers Squibb Company
Summit, NJ 07901 USA
Phone: 1-888-805-4555
US License No. 2252
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
DIN/Aph ID: W0000 00 000000
JOIN: XXXX-XXXXX
LOT: XXXX-XXXXXY
EXP: DD-MMM-YYYY
Bag ID: XX
XXXX-XXXXXY-XX
303400
PRINCIPAL DISPLAY PANEL - 100 mL Cassette Label
idecabtagene vicleucel
Abecma™
NDC 59572-515-03
Genetically modified autologous T cells
FOR AUTOLOGOUS AND INTRAVENOUS USE ONLY
Suspension for IV Infusion
Rx Only
Acceptable Volume 55mL - 100mL per bag
Dosage: See prescribing information and Release for
Infusion Certificate (inside shipper).
Contains: 300 to 460 x 106 anti-BCMA02 CAR-positive T cells
in cryopreservative solution containing 5% DMSO USP.
Dose may be suspended in 1 or more infusion bag(s).
Do not use a leukodepleting filter or irradiate.
Not evaluated for infectious substances. Preservative free.
Store in vapor phase of liquid nitrogen (≤ -130°C).
Mfd by: Celgene Corporation, a Bristol-Myers Squibb Company
Summit, NJ 07901 USA
Phone: 1-888-805-4555
US License No. 2252
First: FIRST NAME
Last: LAST NAME
Date of birth: DD-MMM-YYYY
DIN/Aph ID: W0000 00 000000
JOIN: XXXX-XXXXX
LOT: XXXX-XXXXXY
EXP: DD-MMM-YYYY
Bag ID: XX
XXXX-XXXXXY-XX
303402
| ABECMA
idecabtagene vicleucel suspension |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Celgene Corporation (174201137) |