Drug Detail:Abilify asimtufii (Aripiprazole)
Drug Class:
Highlights of Prescribing Information
ABILIFY ASIMTUFII® (aripiprazole) extended-release injectable suspension, for intramuscular use
Initial U.S. Approval: 2002
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
- Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death (5.1)
- ABILIFY ASIMTUFII is not approved for the treatment of patients with dementia-related psychosis (5.1)
Indications and Usage for Abilify Asimtufii
ABILIFY ASIMTUFII is an atypical antipsychotic indicated:
- for the treatment of schizophrenia in adults (1)
- as maintenance monotherapy treatment of bipolar I disorder in adults (1)
Abilify Asimtufii Dosage and Administration
- For patients naïve to aripiprazole, establish tolerability with oral aripiprazole prior to initiating treatment with ABILIFY ASIMTUFII (2.1)
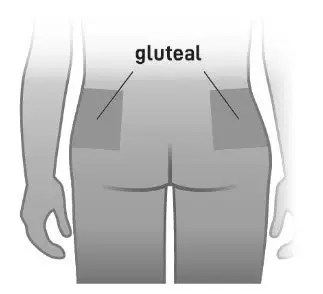
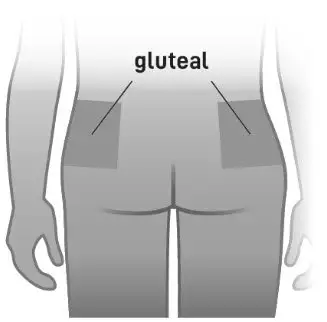
- Administer by intramuscular injection in the gluteal muscle by a healthcare professional. Do not administer by any other route (2.1)
- Recommended dosage is 960 mg administered once every 2 months as a single injection. Dose can be reduced to 720 mg in patients with adverse reactions (2.2)
- Missed doses: Dosage adjustment may be required (2.3)
- Known CYP2D6 poor metabolizers: Recommended dosage is 720 mg administered once every 2 months as a single injection (2.4)
- See Full Prescribing Information for important preparation and administration information (2.5)
Dosage Forms and Strengths
Extended-release injectable suspension: 960 mg/3.2 mL and 720 mg/2.4 mL single-dose pre-filled syringes (3)
Contraindications
Known hypersensitivity to aripiprazole, or to any excipients of ABILIFY ASIMTUFII (4)
Warnings and Precautions
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack, including fatalities) (5.2)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring (5.3)
- Tardive Dyskinesia: Discontinue if clinically appropriate (5.4)
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain (5.5)
- Pathological Gambling and Other Compulsive Behaviors: Consider dose reduction or discontinuation (5.6)
- Orthostatic Hypotension and Syncope: Monitor heart rate and blood pressure and caution in patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope (5.7)
- Leukopenia, Neutropenia, and Agranulocytosis: Perform complete blood counts (CBC) in patients with history of clinically significant low white blood cell count (WBC) or a history of leukopenia or neutropenia. Consider discontinuing ABILIFY ASIMTUFII if clinically significant decline in WBC in the absence of other causative factors (5.9)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold (5.10)
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery (5.11)
Adverse Reactions/Side Effects
Most commonly observed adverse reactions (incidence ≥5% and at least twice the rate of placebo) were increased weight, akathisia, injection site pain, and sedation (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Otsuka America Pharmaceutical, Inc. at 1-800-438-9927 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Dosage adjustments for patients taking CYP2D6 inhibitors, CYP3A4 inhibitors, or CYP3A4 inducers for greater than 14 days (7.1):
| Factors | Dosage Recommendation |
|---|---|
| CYP2D6 Poor Metabolizers taking concomitant CYP3A4 inhibitors | Avoid use |
| Patients taking strong CYP2D6 or CYP3A4 inhibitors | 720 mg |
| Patients taking CYP2D6 and CYP3A4 inhibitors | Avoid use |
| Patients taking CYP3A4 inducers | Avoid use |
Use In Specific Populations
Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2023
Full Prescribing Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. ABILIFY ASIMTUFII is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
1. Indications and Usage for Abilify Asimtufii
ABILIFY ASIMTUFII is indicated:
- for the treatment of schizophrenia in adults
- for maintenance monotherapy treatment of bipolar I disorder in adults
2. Abilify Asimtufii Dosage and Administration
2.1 Important Administration Information
For patients who have never taken aripiprazole, establish tolerability with oral aripiprazole prior to initiating treatment with ABILIFY ASIMTUFII. Due to the half-life of oral aripiprazole, it may take up to 2 weeks to fully assess tolerability.
ABILIFY ASIMTUFII must be administered as an intramuscular gluteal injection by a healthcare professional. Do not administer by any other route.
For detailed preparation and administration instructions, see Dosage and Administration (2.5).
2.2 Recommended Dosage for ABILIFY ASIMTUFII
The recommended dosage of ABILIFY ASIMTUFII is 960 mg, administered once every 2 months (56 days after previous injection).
2.3 Missed Doses
If more than 8 weeks and less than 14 weeks have elapsed since the last injection, administer the next dose of ABILIFY ASIMTUFII as soon as possible. The once every 2 month schedule should be resumed.
If more than 14 weeks have elapsed since the last injection, restart concomitant oral aripiprazole for 14 days with the next administered injection of ABILIFY ASIMTUFII.
2.4 Dosage Recommendations for Cytochrome P450 Considerations
Dosage adjustments for patients who are CYP2D6 poor metabolizers and/or in patients taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors for more than 14 days are described in Table 1.
If the CYP3A4 inhibitor or CYP2D6 inhibitor is withdrawn, the dosage of ABILIFY ASIMTUFII may need to be increased to the previous dose.
Dosage adjustments are not recommended for patients with concomitant use of CYP3A4 inhibitors, CYP2D6 inhibitors or CYP3A4 inducers for less than 14 days.
| Factors | Dosage Recommendation |
|---|---|
| CYP2D6 Poor Metabolizers | |
| Known CYP2D6 Poor Metabolizers | 720 mg once every 2 months |
| Known CYP2D6 Poor Metabolizers taking concomitant CYP3A4 inhibitors | Avoid use |
| Patients Taking 960 mg of ABILIFY ASIMTUFII | |
| Concomitant use of ABILIFY ASIMTUFII with Strong CYP2D6 inhibitors | 720 mg once every 2 months |
| Concomitant use of ABILIFY ASIMTUFII with Strong CYP3A4 inhibitors | 720 mg once every 2 months |
| Concomitant use of ABILIFY ASIMTUFII with Strong CYP2D6 and Strong CYP3A4 inhibitors | Avoid use |
| Concomitant use of ABILIFY ASIMTUFII with CYP3A4 inducers | Avoid use |
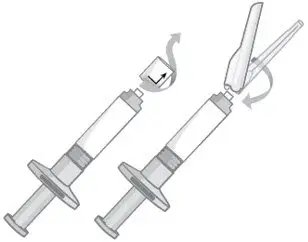
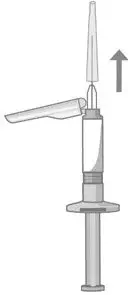
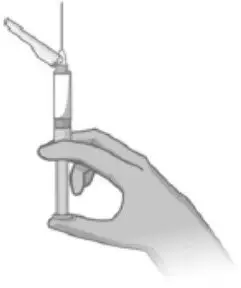
2.5 Preparation and Administration Instructions
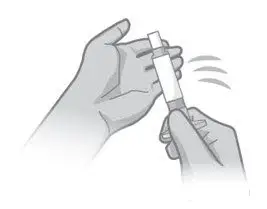
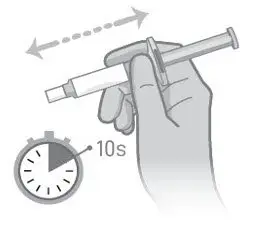
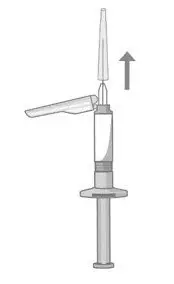
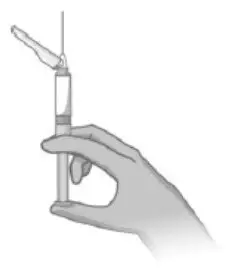
- Read the complete instructions for preparation and administration below and consider referring to the separate Healthcare Provider "Instructions for Use" for additional preparation and administration considerations.
- To be prepared and administered by a healthcare professional only.
- For gluteal intramuscular injection only. Do not administer by any other route.
- Prior to administration, visually inspect ABILIFY ASIMTUFII pre-filled syringe for particulate matter and discoloration. The suspension should appear to be a uniform, homogeneous suspension that is opaque and milky-white in color. Do not use ABILIFY ASIMTUFII pre-filled syringe if the suspension is discolored, or particulate matter is present
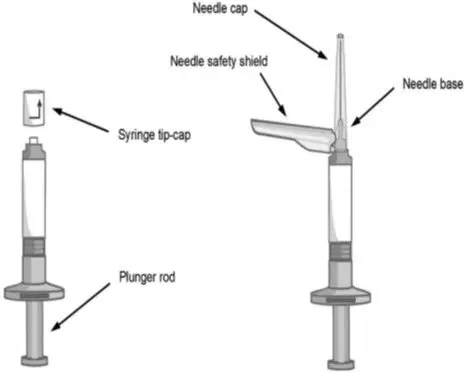
- Each kit contains one sterile pre-filled syringe containing ABILIFY ASIMTUFII 720 mg or 960 mg and two safety needles:
- One sterile 1 ½ inch, 22 gauge needle (in black packaging)
- One sterile 2 inch, 21 gauge needle (in green packaging)
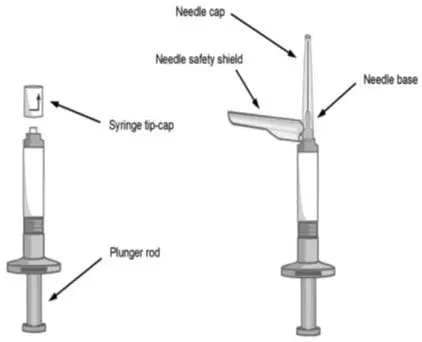
- Each kit contains one sterile pre-filled syringe containing ABILIFY ASIMTUFII 720 mg or 960 mg and two safety needles:
3. Dosage Forms and Strengths
Extended-release injectable suspension: sterile, white to off-white, aqueous suspension in a single-dose, pre-filled syringe.
| Dose Strength | Volume | Label Color | Syringe Tip Wrap |
|---|---|---|---|
| 720 mg | 2.4 mL | Light Blue | Aqua |
| 960 mg | 3.2 mL | Pink | Light Blue |
4. Contraindications
ABILIFY ASIMTUFII is contraindicated in patients with a known hypersensitivity to aripiprazole, or any of the excipients. Hypersensitivity reactions ranging from pruritus/urticaria to anaphylaxis have been reported in patients receiving aripiprazole [see Adverse Reactions (6.1)].
5. Warnings and Precautions
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear.
ABILIFY ASIMTUFII is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions (5.2)].
5.2 Cerebrovascular Adverse Reactions, Including Stroke in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled clinical studies (two flexible-dose and one fixed-dose study) of dementia-related psychosis, there was an increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack), including fatalities, in oral aripiprazole-treated patients (mean age: 84 years; range: 78 to 88 years). In the fixed-dose study, there was a statistically significant dose response relationship for cerebrovascular adverse reactions in patients treated with oral aripiprazole. ABILIFY ASIMTUFII is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
5.3 Neuroleptic Malignant Syndrome
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex has been reported with antipsychotic drugs, including aripiprazole. Rare cases of NMS have been reported during aripiprazole treatment in the global clinical database.
Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis) and acute renal failure.
If NMS is suspected, immediately discontinue ABILIFY ASIMTUFII and provide symptomatic treatment and monitoring.
5.4 Tardive Dyskinesia
Tardive dyskinesia, a syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible appear to increase as the duration of treatment and the total cumulative dose increases. The syndrome can develop, after relatively brief treatment periods at low doses. It may also occur after discontinuation of treatment.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of the syndrome and may mask the underlying process. The effect of symptomatic suppression on the long-term course of the syndrome is unknown.
Given these considerations, ABILIFY ASIMTUFII should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that 1) is known to respond to antipsychotic drugs and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient treated with ABILIFY ASIMTUFII, drug discontinuation should be considered. However, some patients may require treatment with ABILIFY ASIMTUFII despite the presence of the syndrome.
5.5 Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes including hyperglycemia/diabetes mellitus, dyslipidemia, and body weight gain. While all drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
5.6 Pathological Gambling and Other Compulsive Behaviors
Post-marketing case reports suggest that patients can experience intense urges, particularly for gambling, and the inability to control these urges while taking aripiprazole. Other compulsive urges, reported less frequently, include: sexual urges, shopping, eating or binge eating, and other impulsive or compulsive behaviors. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or intense gambling urges, compulsive sexual urges, compulsive shopping, binge or compulsive eating, or other urges while being treated with aripiprazole. It should be noted that impulse-control symptoms can be associated with the underlying disorder. In some cases, although not all, urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Compulsive behaviors may result in harm to the patient and others if not recognized. Consider dose reduction or stopping the medication if a patient develops such urges.
5.7 Orthostatic Hypotension and Syncope
ABILIFY ASIMTUFII may cause orthostatic hypotension, perhaps due to its α1-adrenergic receptor antagonism. Associated reactions related to orthostatic hypotension can include dizziness, tachycardia, and in some patients, syncope. In the short-term, placebo-controlled trial in adults with schizophrenia, the adverse reaction of presyncope was reported in 1/167 (0.6%) of patients treated with Abilify Maintena (once monthly dosing), while syncope and orthostatic hypotension were each reported in 1/172 (0.6%) of patients treated with placebo. During the stabilization phase of the randomized-withdrawal (maintenance) study in adult patients with schizophrenia, orthostasis-related adverse events were reported in 4/576 (0.7%) of patients treated with Abilify Maintena, including abnormal orthostatic blood pressure (1/576, 0.2%), postural dizziness (1/576, 0.2%), presyncope (1/576, 0.2%) and orthostatic hypotension (1/576, 0.2%).
In the short-term placebo-controlled trial of Abilify Maintena in adults with schizophrenia, there were no patients in either treatment group with a significant orthostatic change in blood pressure (defined as a decrease in systolic blood pressure ≥20 mmHg accompanied by an increase in heart rate ≥25 bpm when comparing standing to supine values). During the stabilization phase of the randomized-withdrawal (maintenance) study in adult patients with schizophrenia, the incidence of significant orthostatic change in blood pressure was 0.2% (1/575).
Use ABILIFY ASIMTUFII with caution in patients with known cardiovascular disease (heart failure, history of myocardial infarction or ischemia, conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient to hypotension (e.g., dehydration, hypovolemia, and treatment with antihypertensive medications). Monitoring of orthostatic vital signs should be considered in patients who are vulnerable to hypotension.
5.8 Falls
Antipsychotics, including ABILIFY ASIMTUFII, may cause somnolence, postural hypotension, motor and sensory instability which may lead to falls and, consequently, fractures or other fall-related injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.9 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trials and post-marketing experience, leukopenia and neutropenia have been reported temporally related to antipsychotic agents, including aripiprazole. Agranulocytosis has also been reported [see Adverse Reactions (6.1)].
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) absolute neutrophil count (ANC) and a history of drug-induced leukopenia/neutropenia. In patients with a history of a clinically significant low WBC/ANC or drug-induced leukopenia/neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of ABILIFY ASIMTUFII at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue ABILIFY ASIMTUFII in patients with severe neutropenia (ANC<1000/mm3) and follow their WBC counts until recovery.
5.10 Seizures
As with other antipsychotic drugs, use ABILIFY ASIMTUFII cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
5.11 Potential for Cognitive and Motor Impairment
ABILIFY ASIMTUFII, like other antipsychotics, may impair judgment, thinking, or motor skills. Patients should be cautioned about performing activities that require mental alertness such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that therapy with ABILIFY ASIMTUFII does not affect them adversely.
5.12 Body Temperature Regulation
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing ABILIFY ASIMTUFII for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, (e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration).
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia - Related Psychosis Use [see Boxed Warning and Warnings and Precautions (5.1)]
- Cerebrovascular Adverse Reactions, Including Stroke in Elderly Patients with Dementia-Related Psychosis [see Warnings and Precautions (5.2)]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.3)]
- Tardive Dyskinesia [see Warnings and Precautions (5.4)]
- Metabolic Changes [see Warnings and Precautions (5.5)]
- Pathological Gambling and Other Compulsive Behaviors [see Warnings and Precautions (5.6)]
- Orthostatic Hypotension and Syncope [see Warnings and Precautions (5.7)]
- Falls [see Warnings and Precautions (5.8)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.9)]
- Seizures [see Warnings and Precautions (5.10)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.11)]
- Body Temperature Regulation [see Warnings and Precautions (5.12)]
- Dysphagia [see Warnings and Precautions (5.13)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ABILIFY ASIMTUFII for the treatment of schizophrenia in adults and maintenance monotherapy treatment of bipolar I disorder in adults is based on adequate and well-controlled studies of Abilify Maintena. The safety data from those studies is presented below.
Injection Site Reactions with ABILIFY ASIMTUFII
ABILIFY ASIMTUFII was evaluated in 266 patients with schizophrenia or bipolar I disorder in an open-label, multiple-dose, randomized, parallel-arm multi-center study.
The percentage of patients in the open-label study reporting any injection site-related adverse reactions (all reported as injection site pain) was 19% for patients treated with ABILIFY ASIMTUFII 960 mg and 9% for patients treated with Abilify Maintena 400 mg. In both treatment groups, the majority of the injection site pain events coincided with the first injection of ABILIFY ASIMTUFII 960 mg (21/24 patients) or Abilify Maintena 400 mg (7/12 patients), was reported with decreasing frequency upon subsequent injections. The overall mean visual analog scale scores (0=no pain to 100=unbearably painful) for patient reported rating of pain were similar in both treatment groups at the last injection: 0.8 pre-dose and 1.4 post-dose for the ABILIFY ASIMTUFII 960 mg group compared to 1.3 post-dose for the Abilify Maintena 400 mg group.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of aripiprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: occurrences of allergic reaction (anaphylactic reaction, angioedema, laryngospasm, pruritus/urticaria, or oropharyngeal spasm), blood glucose fluctuation, drug reaction with eosinophilia and systemic symptoms (DRESS), hiccups, pathological gambling.
7. Drug Interactions
7.1 Drugs Having Clinically Important Interactions with ABILIFY ASIMTUFII
Table 7 presents clinically significant drug interactions with ABILIFY ASIMTUFII.
| Strong CYP3A4 Inhibitors AND/OR strong CYP2D6 inhibitors | |
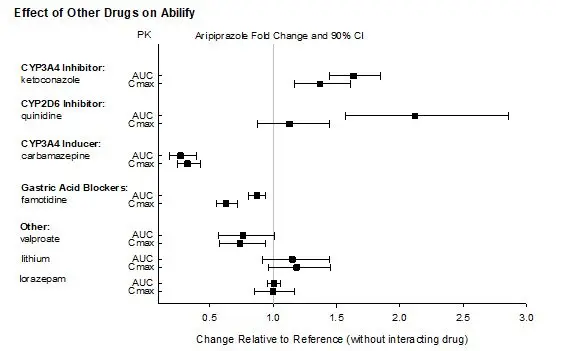
| Clinical Rationale | Concomitant use of oral aripiprazole with strong CYP3A4 AND/OR CYP2D6 inhibitors increased the exposure of aripiprazole [see Clinical Pharmacology (12.3)]. |
| Clinical Recommendation | Concomitant use of a strong CYP3A4 inhibitor OR a strong CYP2D6 inhibitor
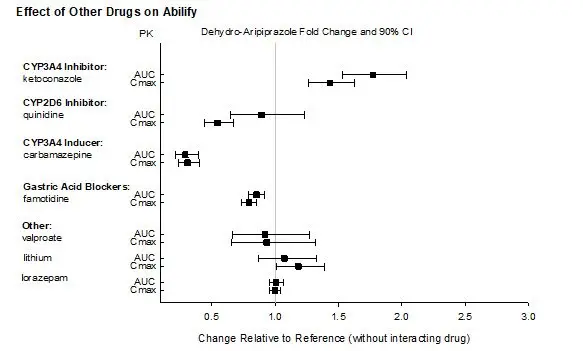
Reduce the dosage of ABILIFY ASIMTUFII when administered concomitantly with a strong CYP3A4 inhibitor OR a strong CYP2D6 inhibitor for more than 14 days [see Dosage and Administration (2.4)]. Concomitant Use of a strong CYP3A4 inhibitor AND a strong CYP2D6 inhibitor Avoid use of ABILIFY ASIMTUFII when administered concomitantly with a strong CYP3A4 inhibitor AND a strong CYP2D6 inhibitor for more than 14 days [see Dosage and Administration (2.4)]. |
| Strong CYP3A4 Inducers | |
| Clinical Rationale | Concomitant use of oral aripiprazole and carbamazepine decreased the exposure of aripiprazole [see Clinical Pharmacology (12.3)]. |
| Clinical Recommendation | Avoid use of ABILIFY ASIMTUFII in combination with a strong CYP3A4 inducer (e.g., carbamazepine) for greater than 14 days [see Dosage and Administration (2.4)]. |
| Antihypertensive Drugs | |
| Clinical Rationale | Due to its alpha-adrenergic antagonism, aripiprazole has the potential to enhance the effect of certain antihypertensive agents. |
| Clinical Recommendation | Monitor blood pressure and adjust dose accordingly [see Warnings and Precautions (5.7)]. |
| Benzodiazepines | |
| Clinical Rationale | The intensity of sedation was greater with the combination of oral aripiprazole and lorazepam as compared to that observed with aripiprazole alone. The orthostatic hypotension observed was greater with the combination as compared to that observed with lorazepam alone [see Warnings and Precautions (5.7)]. |
| Clinical Recommendation | Monitor sedation and blood pressure. Adjust dose accordingly. |
7.2 Drugs Having No Clinically Important Interactions with ABILIFY ASIMTUFII
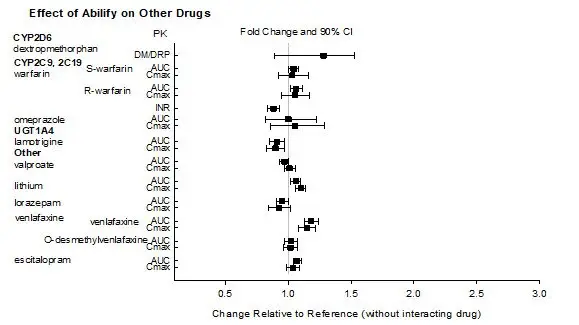
Based on pharmacokinetic studies with oral aripiprazole, no dosage adjustment of ABILIFY ASIMTUFII is required when administered concomitantly with famotidine, valproate, lithium, lorazepam [see Clinical Pharmacology (12.3)].
In addition, no dosage adjustment is necessary for substrates of CYP2D6, CYP2C9, CYP2C19, or CYP3A4 when coadministered with ABILIFY ASIMTUFII. Additionally, no dosage adjustment is necessary for valproate, lithium, lamotrigine, lorazepam, or sertraline when coadministered with ABILIFY ASIMTUFII [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of ABILIFY ASIMTUFII in pediatric patients have not been established.
8.5 Geriatric Use
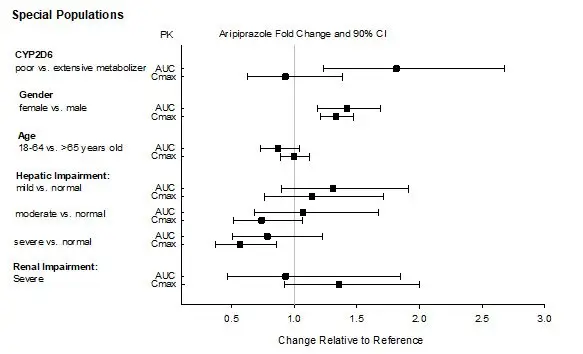
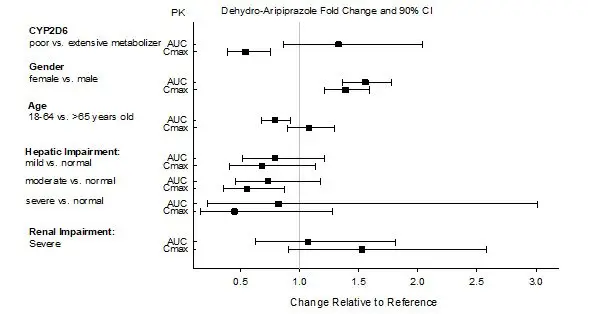
Clinical studies of ABILIFY ASIMTUFII did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience and pharmacokinetic data have not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
In single-dose and multiple-dose pharmacokinetic studies with oral aripiprazole, there was no detectable age effect in the population pharmacokinetic analysis in schizophrenia patients. No dosage adjustments are recommended based on age alone. ABILIFY ASIMTUFII is not approved for the treatment of patients with dementia-related psychosis [see also Boxed Warning and Warnings and Precautions (5.1)].
8.6 CYP2D6 Poor Metabolizers
Dosage adjustment is recommended in known CYP2D6 poor metabolizers due to high aripiprazole concentrations. Approximately 8% of Caucasians and 3% to 8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM) [see Dosage and Administration (2.4)].
11. Abilify Asimtufii Description
Aripiprazole is an atypical antipsychotic which is present in ABILIFY ASIMTUFII as its monohydrate polymorphic form. Aripiprazole monohydrate is 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl] butoxy]-3,4 dihydrocarbostyril monohydrate. The empirical formula is C23H27Cl2N3O2∙H2O and its molecular weight is 466.40. The chemical structure is:
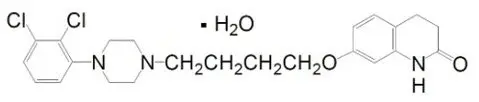
ABILIFY ASIMTUFII (aripiprazole) is available as a white to off-white, sterile, aqueous extended-release suspension for intramuscular injection in 720 mg or 960 mg dose strength, pre-filled syringes. The labeled strengths are calculated based on the anhydrous form (aripiprazole). Inactive ingredients are carboxymethylcellulose sodium (5 mg/mL), polyethylene glycol 400 (1 mg/mL), povidone (4 mg/mL), sodium chloride (6.1 mg/mL), sodium phosphate monobasic monohydrate (0.74 mg/mL), sodium hydroxide (to adjust pH) and water for injection (q.s.).
12. Abilify Asimtufii - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of aripiprazole in the treatment of schizophrenia and bipolar I disorder is unknown.
The efficacy of aripiprazole could be mediated through a combination of partial agonist activity at dopamine D2 and serotonin 5-HT1A receptors and antagonist activity at 5-HT2A receptors.
12.2 Pharmacodynamics
Aripiprazole exhibits high affinity for dopamine D2 and D3 (Kis 0.34 and 0.8 nM, respectively), serotonin 5-HT1A and 5-HT2A receptors (Kis 1.7 and 3.4 nM, respectively), moderate affinity for dopamine D4, serotonin 5-HT2C and 5-HT7, alpha1-adrenergic and histamine H1 receptors (Kis of 44 nM, 15 nM, 39 nM, 57 nM, and 61 nM, respectively), and moderate affinity for the serotonin reuptake site (Ki=98 nM). Aripiprazole has no appreciable affinity for cholinergic muscarinic receptors (IC50>1000 nM). Actions at receptors other than D2, 5-HT1A, and 5-HT2A could explain some of the other adverse reactions of aripiprazole (e.g., the orthostatic hypotension observed with aripiprazole may be explained by its antagonist activity at adrenergic alpha1 receptors).
12.3 Pharmacokinetics
ABILIFY ASIMTUFII activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents about 29% of the parent drug exposure in plasma.
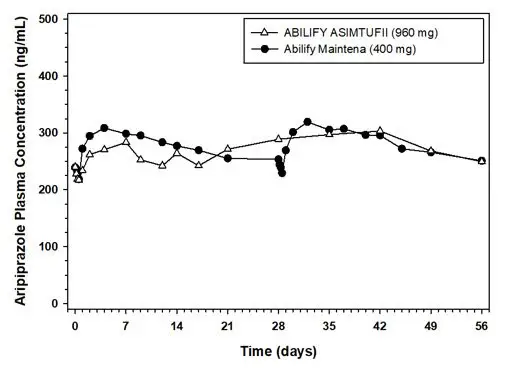
ABILIFY ASIMTUFII delivers aripiprazole over a 2-month period. ABILIFY ASIMTUFII has linear PK in the approved dose range. Steady-state aripiprazole exposures were reached by the fourth dose. Plasma exposures at steady state were compared between ABILIFY ASIMTUFII (960 mg, once every 2 months) and Abilify Maintena (400 mg, once every month). The average plasma concentrations (Cavg) of aripiprazole were 263 ng/mL and 280 ng/mL for ABILIFY ASIMTUFII and Abilify Maintena, respectively. The Cmax of aripiprazole were 342 ng/mL and 344 ng/mL for ABILIFY ASIMTUFII and Abilify Maintena, respectively.
Figure 9: Mean Plasma Concentration of Aripiprazole Following the Fourth Administration of ABILIFY ASIMTUFII 960 mg versus the Seventh and Eighth Administration of Abilify Maintena 400 mg
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
The toxicological profile for aripiprazole administered to experimental animals by intramuscular injection is generally similar to that seen following oral administration at comparable plasma levels of the drug. In dogs, repeated intramuscular dosing of the 2-month aripiprazole extended release injectable suspension over a period of 52 weeks produced no clinical evidence of significant local irritation, and resulted in slight foreign-body type of localized granulomatous inflammatory reaction to deposited drug at the injection site. These effects gradually resolved with discontinuation of dosing.
Oral aripiprazole produced retinal degeneration in albino rats in a 26-week chronic toxicity study at a dose of 60 mg/kg and in a 2-year carcinogenicity study at doses of 40 and 60 mg/kg. The 40 and 60 mg/kg/day doses are 13 and 19 times the maximum recommended human oral dose (MRHD) based on mg/m2 body surface and 7 to 14 times human exposure at the oral MRHD based on AUC. Evaluation of the retinas of albino mice and of monkeys did not reveal evidence of retinal degeneration. Additional studies to further evaluate the mechanism have not been performed. The relevance of this finding to human risk is unknown.
14. Clinical Studies
14.1 Schizophrenia
The efficacy of ABILIFY ASIMTUFII (once every 2 month dosing) for the treatment of schizophrenia in adults is based on adequate and well-controlled studies of Abilify Maintena (once monthly dosing). The results of these adequate and well-controlled studies are presented below.
The efficacy of Abilify Maintena (once monthly dosing) for treatment of schizophrenia was established in:
• One short-term (12-week), randomized, double-blind, placebo-controlled trial in acutely relapsed adults (Study 1)
• One longer-term, double-blind, placebo-controlled, randomized-withdrawal (maintenance) trial in adults (Study 2).
Short-Term Efficacy
In the short-term (12-week), randomized, double-blind, placebo-controlled trial in acutely relapsed adults (Study 1), the primary measure used for assessing psychiatric signs and symptoms was the Positive and Negative Syndrome Scale (PANSS). The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210. The primary endpoint was the change from baseline in PANSS total score to week 10.
The inclusion criteria for this short-term trial included adult inpatients who met DSM-IV-TR criteria for schizophrenia. In addition, all patients entering the trial must have experienced an acute psychotic episode as defined by both PANSS Total Score ≥80 and a PANSS score of >4 on each of four specific psychotic symptoms (conceptual disorganization, hallucinatory behavior, suspiciousness/persecution, unusual thought content) at screening and baseline. The key secondary endpoint was the change from baseline in Clinical Global Impression-Severity (CGI-S) assessment scale to week 10. The CGI-S rates the severity of mental illness on a scale of 1 (normal) to 7 (among the most extremely ill) based on the total clinical experience of the rater in treating patients with schizophrenia. Patients had a mean PANSS total score of 103 (range 82 to 144) and a CGI-S score of 5.2 (markedly ill) at entry.
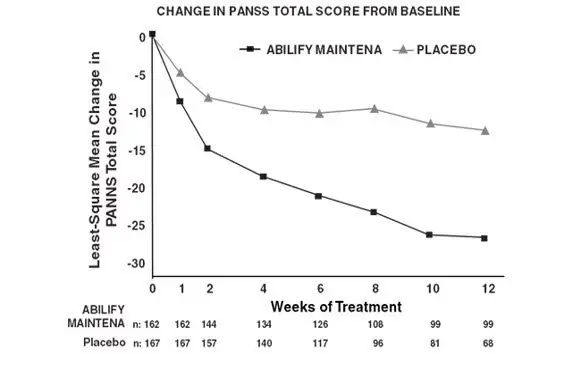
In this 12-week study (n=339) comparing Abilify Maintena (n=167) to placebo (n=172), patients were administered 400 mg Abilify Maintena or placebo on days 0, 28, and 56. The dose could be adjusted down and up within the range of 400 to 300 mg on a one-time basis. Abilify Maintena was superior to placebo in improving the PANSS total score at the end of week 10 (see Table 8).
| Primary Efficacy Measure: PANSS Total Score | ||||
|---|---|---|---|---|
| Study Number | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference*
(95% CI) |
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval. | ||||
|
||||
| Study 1 | Abilify Maintena (400 to 300 mg) | 102.4 (11.4) | -26.8 (1.6) | -15.1 (-19.4, -10.8) |
| Placebo | 103.4 (11.1) | -11.7 (1.6) | -- | |
The change in PANSS total score by week is shown in Figure 15. Abilify Maintena also showed improvement in symptoms represented by CGI-S score mean change from baseline to week 10. The results of exploratory subgroup analyses by gender, race, age, ethnicity, and BMI were similar to the results of the overall population.
| n = the number of patients remaining in the respective study arm at each time point |
| Figure 15: Weekly PANSS Total Score-Change in the 12-Week, Placebo-Controlled Study with Abilify Maintena in Schizophrenia - Study 1 (Adults) |
 |
14.2 Bipolar I Disorder - Maintenance Monotherapy
The efficacy of ABILIFY ASIMTUFII (once every 2 month dosing) for the treatment of maintenance monotherapy treatment of bipolar I disorder in adults is based on an adequate and well-controlled study of Abilify Maintena (once monthly dosing). The results of the adequate and well-controlled study are presented below.
The efficacy of Abilify Maintena (once monthly dosing) for the maintenance treatment of bipolar I disorder was established in a 52-week, double-blind, placebo-controlled, randomized withdrawal trial in adult patients who were experiencing a manic episode at trial entry, met DSM-IV-TR criteria for bipolar I disorder, and had a history of at least one previous manic or mixed episode with manic symptoms of sufficient severity to require one of the following interventions: hospitalization and/or treatment with a mood stabilizer, and/or treatment with an antipsychotic agent.
Clinical ratings during this trial included:
- Young Mania Rating Scale (YMRS)-an 11-item, clinician-rated scale used to assess the degree of manic symptomatology, in a range with 0 representing no symptoms, and 60 representing worst symptoms; Montgomery-Asberg Depression Rating Scale (MADRS) – a 10-item clinician-related scale used to assess the degree of depressive symptomatology, with 1 representing no symptoms, and 60 representing worst symptoms; Clinical Global Impression Bipolar Version Severity of Illness (CGI-BP-S) a scale of 1 (normal, not at all ill) to 7 (very severely ill patient) based on the patient's severity of illness mania, depression, and overall bipolar illness.
This trial included:
- A 4 to 6 week, open-label, oral conversion phase for patients on treatments for bipolar I disorder other than aripiprazole. A total of 466 patients entered this phase.
- A 2 to 8 week, open-label, oral aripiprazole stabilization phase (target dose of 15 mg to 30 mg once daily). A total of 632 patients entered this phase. Patients were 18 to 65 years old (mean 40.7 years) and 60% were female. The mean (range) baseline scores were: YMRS total, 16.9 MADRS total, 5.7, and CGI-BP-S overall, 3.4 (mildly to moderately ill). Prior to the next phase, stabilization was required. Stabilization was defined as having all of the following at one bi-weekly visit: Outpatient status, YMRS total score ≤12, MADRS total score ≤12 no active suicidality; with active suicidality defined as a score of 4 or more on the MADRS item 10 OR an answer of "yes" on question 4 or 5 on the Columbia Suicide Severity Rating Scale (C-SSRS).
- A minimum 12-week, uncontrolled, single-blind Abilify Maintena stabilization phase (treatment with 400 mg of Abilify Maintena given every 4 weeks in conjunction with oral aripiprazole [10 mg to 20 mg/day] for the first 2 weeks). The dose of Abilify Maintena was allowed to be decreased to 300 mg due to adverse reactions. A total of 425 patients entered this phase. The mean (range) baseline scores were: YMRS total, 5.8, MADRS total 3.7, and CGI-BP-S overall, 2.1 (minimally ill). Prior to the next phase, stabilization was required (see above for the definition of stabilization) for 8 consecutive weeks starting at week 6.
- A double-blind, placebo-controlled, randomized-withdrawal phase to observe for recurrence to a mood episode (defined below) for up to 52 weeks. A total of 266 patients were randomized 1:1 to the same dose of Abilify Maintena they were receiving at the end of the stabilization phase, (400 mg or 300 mg administered once every 4 weeks) or placebo. The mean (range) baseline scores were: YMRS total, 2.8 (0 to 12), MADRS total, 2.7 (0 to 12), and CGI-S overall, 1.7 (minimally ill). The dose could be decreased to 300 mg for tolerability and returned once to 400 mg.
The primary efficacy endpoint was time from randomization to recurrence of any mood episode. Recurrence was defined as the first occurrence of one or more of the following criteria:
1) Hospitalization for any mood episode OR 2) Any of the following: a. YMRS total score ≥15 OR b. MADRS total score ≥15 OR c. Clinical Global Impression - Bipolar Version-Severity (CGI-BP-S) score >4 (overall score) OR 3) Serious adverse event (SAE) of worsening disease (bipolar I disorder) OR 4) Discontinuation due to lack of efficacy or discontinuation due to an adverse event (AE) of worsening disease OR 5) Clinical worsening with the need for addition of a mood stabilizer, antidepressant treatment, antipsychotic medication, and/or increase greater than the allowed benzodiazepine doses for treatment of symptoms of an underlying mood disorder OR 6) Active suicidality, which is defined as a score of 4 or more on the MADRS item 10 OR an answer of "yes" on question 4 or 5 on the C-SSRS
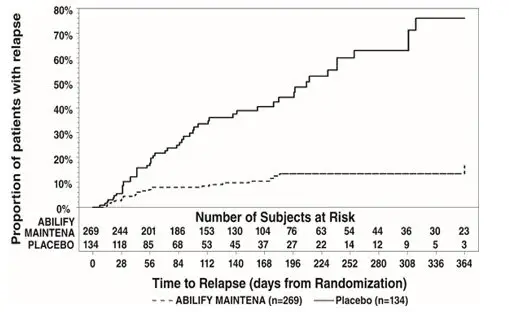
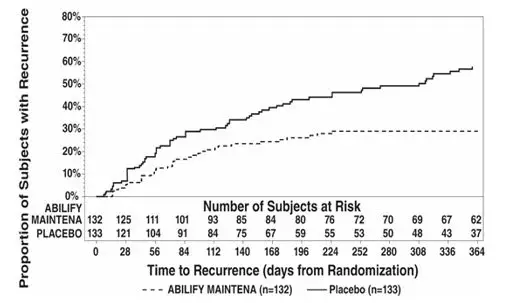
Analysis demonstrated a statistically significantly longer time to recurrence of any mood episode in subjects randomized to the Abilify Maintena group than compared to placebo-treated subjects. The Kaplan-Meier curves of the time of recurrence to any mood episode during the double-blind treatment phase for Abilify Maintena and placebo groups are shown in Figure 17.
|
| Figure 17: Kaplan-Meier Estimation of Cumulative Recurrence Rate for Any Mood Episode* in Abilify Maintena-Treated Adults |
 |
Analysis by type of mood recurrence demonstrated a statistically significantly longer time to recurrence for both manic and mixed mood episodes in subjects treated with Abilify Maintena compared to those treated with placebo. There was no substantial difference between treatment groups in delaying time to recurrence of depressive mood episodes.
An examination of subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex, or race.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Issued: 4/2023 | |||
| MEDICATION GUIDE ABILIFY ASIMTUFII® (a-BIL-i-fy AH-SIM-TUH-FYE) (aripiprazole) for extended-release injectable suspension, for intramuscular use |
||||
| What is the most important information I should know about ABILIFY ASIMTUFII? ABILIFY ASIMTUFII may cause serious side effects, including:
|
||||
| What is ABILIFY ASIMTUFII?
ABILIFY ASIMTUFII is a prescription medicine given by injection by a healthcare provider:
|
||||
| Do not receive ABILIFY ASIMTUFII if you are allergic to aripiprazole or any of the ingredients in ABILIFY ASIMTUFII. See the end of this Medication Guide for a complete list of ingredients in ABILIFY ASIMTUFII. | ||||
Before receiving ABILIFY ASIMTUFII, tell your healthcare provider about all of your medical conditions, including if you:
ABILIFY ASIMTUFII and other medicines may affect each other causing possible serious side effects. ABILIFY ASIMTUFII may affect the way other medicines work, and other medicines may affect how ABILIFY ASIMTUFII works. Your healthcare provider can tell you if it is safe to receive ABILIFY ASIMTUFII with your other medicines. Do not start or stop any medicines during treatment with ABILIFY ASIMTUFII without first talking to your healthcare provider. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. |
||||
How should I receive ABILIFY ASIMTUFII?
|
||||
What should I avoid while receiving ABILIFY ASIMTUFII?
|
||||
| What are the possible side effects of ABILIFY ASIMTUFII? ABILIFY ASIMTUFII may cause serious side effects, including:
|
||||
|
|
|||
|
||||
|
||||
|
| |||
|
||||
These are not all the possible side effects of ABILIFY ASIMTUFII. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
| General information about the safe and effective use of ABILIFY ASIMTUFII.
If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about ABILIFY ASIMTUFII that is written for healthcare professionals. |
||||
| What are the ingredients in ABILIFY ASIMTUFII?
Active ingredient: aripiprazole monohydrate Inactive ingredients: carboxymethylcellulose sodium, polyethylene glycol 400, povidone, sodium chloride, sodium phosphate monobasic monohydrate, sodium hydroxide and water for injection. ABILIFY ASIMTUFII is a trademark of Otsuka Pharmaceutical Co., Ltd. ©2023, Otsuka Pharmaceutical Co., Ltd., 2-9 Kanda-Tsukasamachi, Chiyoda-ku, Tokyo, 101-8535 Japan For more information about ABILIFY ASIMTUFII, go to www.ABILIFY.com or call 1-800-441-6763. |
||||
| ABILIFY ASIMTUFII
aripiprazole injection, suspension, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ABILIFY ASIMTUFII
aripiprazole injection, suspension, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Otsuka America Pharmaceutical, Inc (008314390) |