Drug Detail:Actemra (Tocilizumab)
Drug Class: Interleukin inhibitors
Highlights of Prescribing Information
ACTEMRA® (tocilizumab) injection, for intravenous or subcutaneous use
Initial U.S. Approval: 2010
WARNING: RISK OF SERIOUS INFECTIONS
See full prescribing information for complete boxed warning.
- Serious infections leading to hospitalization or death including tuberculosis (TB), bacterial, invasive fungal, viral, and other opportunistic infections have occurred in patients receiving ACTEMRA. (5.1)
- If a serious infection develops, interrupt ACTEMRA until the infection is controlled. (5.1)
- Perform test for latent TB (except patients with COVID-19); if positive, start treatment for TB prior to starting ACTEMRA. (5.1)
- Monitor all patients for active TB during treatment, even if initial latent TB test is negative. (5.1)
Recent Major Changes
| Indications and Usage (1.7) | 12/2022 |
| Dosage and Administration (2.1, 2.3, 2.9, 2.11) | 02/2022 |
| Dosage and Administration (2.1, 2.8, 2.9) | 12/2022 |
| Warnings and Precautions (5.1, 5.3, 5.4) | 12/2022 |
Indications and Usage for Actemra
ACTEMRA® (tocilizumab) is an interleukin-6 (IL-6) receptor antagonist indicated for treatment of:
Rheumatoid Arthritis (RA) (1.1)
- Adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
Giant Cell Arteritis (GCA) (1.2)
- Adult patients with giant cell arteritis.
Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) (1.3)
- Slowing the rate of decline in pulmonary function in adult patients with systemic sclerosis-associated interstitial lung disease (SSc-ILD)
Polyarticular Juvenile Idiopathic Arthritis (PJIA) (1.4)
- Patients 2 years of age and older with active polyarticular juvenile idiopathic arthritis.
Systemic Juvenile Idiopathic Arthritis (SJIA) (1.5)
- Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
Cytokine Release Syndrome (CRS) (1.6)
- Adults and pediatric patients 2 years of age and older with chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome.
Coronavirus Disease 2019 (COVID-19) (1.7)
- Hospitalized adult patients with coronavirus disease 2019 (COVID-19) who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Actemra Dosage and Administration
For RA, pJIA and sJIA, ACTEMRA may be used alone or in combination with methotrexate: and in RA, other DMARDs may be used. (2)
General Administration and Dosing Information (2.1)
- RA, GCA, SSc-ILD, PJIA and SJIA – It is recommended that ACTEMRA not be initiated in patients with an absolute neutrophil count (ANC) below 2000 per mm3, platelet count below 100,000 per mm3, or ALT or AST above 1.5 times the upper limit of normal (ULN)(5.3, 5.4).
- COVID-19 – It is recommended that ACTEMRA not be initiated in patients with an absolute neutrophil count (ANC) below 1000 per mm3, platelet count below 50,000 mm3, or ALT or AST above 10 times ULN (5.3, 5.4).
- In RA, CRS or COVID-19 patients, ACTEMRA doses exceeding 800 mg per infusion are not recommended. (2.2, 2.7, 12.3)
- In GCA patients, ACTEMRA doses exceeding 600 mg per infusion are not recommended. (2.3, 12.3)
Rheumatoid Arthritis (2.2)
Recommended Adult Intravenous Dosage:
When used in combination with DMARDs or as monotherapy the recommended starting dose is 4 mg per kg every 4 weeks followed by an increase to 8 mg per kg every 4 weeks based on clinical response.
Recommended Adult Subcutaneous Dosage:
| Patients less than 100 kg weight | 162 mg administered subcutaneously every other week, followed by an increase to every week based on clinical response |
| Patients at or above 100 kg weight | 162 mg administered subcutaneously every week |
Giant Cell Arteritis (2.3)
Recommended Adult Intravenous Dosage:
The recommended dose is 6 mg per kg every 4 weeks in combination with a tapering course of glucocorticoids. ACTEMRA can be used alone following discontinuation of glucocorticoids.
Recommended Adult Subcutaneous Dosage:
The recommended dose is 162 mg given once every week as a subcutaneous injection, in combination with a tapering course of glucocorticoids.
A dose of 162 mg given once every other week as a subcutaneous injection, in combination with a tapering course of glucocorticoids, may be prescribed based on clinical considerations.
ACTEMRA can be used alone following discontinuation of glucocorticoids.
Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD) (2.4)
Recommended Adult Subcutaneous Dosage:
The recommended dose of ACTEMRA for adult patients with SSc-ILD is 162 mg given once every week as a subcutaneous injection.
Polyarticular Juvenile Idiopathic Arthritis (2.5)
| Recommended Intravenous PJIA Dosage Every 4 Weeks | |
|---|---|
| Patients less than 30 kg weight | 10 mg per kg |
| Patients at or above 30 kg weight | 8 mg per kg |
| Recommended Subcutaneous PJIA Dosage | |
|---|---|
| Patients less than 30 kg weight | 162 mg once every three weeks |
| Patients at or above 30 kg weight | 162 mg once every two weeks |
Systemic Juvenile Idiopathic Arthritis (2.6)
| Recommended Intravenous SJIA Dosage Every 2 Weeks | |
|---|---|
| Patients less than 30 kg weight | 12 mg per kg |
| Patients at or above 30 kg weight | 8 mg per kg |
| Recommended Subcutaneous SJIA Dosage | |
|---|---|
| Patients less than 30 kg weight | 162 mg every two weeks |
| Patients at or above 30 kg weight | 162 mg every week |
Cytokine Release Syndrome (2.7)
| Recommended Intravenous CRS Dosage | |
|---|---|
| Patients less than 30 kg weight | 12 mg per kg |
| Patients at or above 30 kg weight | 8 mg per kg |
| Alone or in combination with corticosteroids. | |
Coronavirus Disease 2019 (2.8)
The recommended dosage of ACTEMRA for adult patients with COVID-19 is 8 mg per kg administered by a 60-minute intravenous infusion.
Administration of Intravenous formulation (2.9)
- For patients with RA, GCA, COVID-19, CRS, PJIA, and SJIA patients at or above 30 kg, dilute to 100 mL in 0.9% or 0.45% Sodium Chloride Injection, USP for intravenous infusion using aseptic technique.
- For PJIA, SJIA and CRS patients less than 30 kg, dilute to 50 mL in 0.9% or 0.45% Sodium Chloride Injection, USP for intravenous infusion using aseptic technique.
- Administer as a single intravenous drip infusion over 1 hour; do not administer as bolus or push.
Administration of Subcutaneous formulation (2.10)
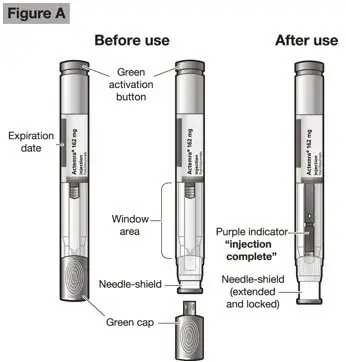
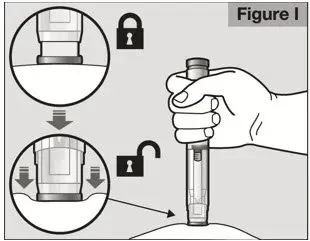
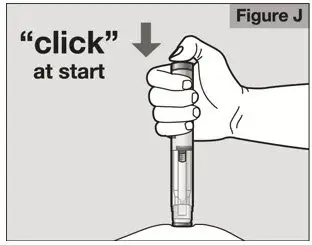
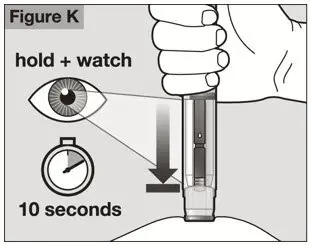
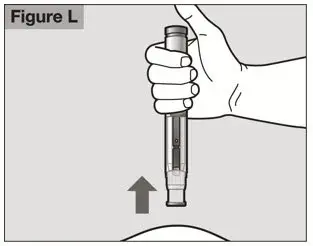
- Follow the Instructions for Use for prefilled syringe and prefilled ACTPen® autoinjector
Dose Modifications (2.11)
- Recommended for management of certain dose-related laboratory changes including elevated liver enzymes, neutropenia, and thrombocytopenia.
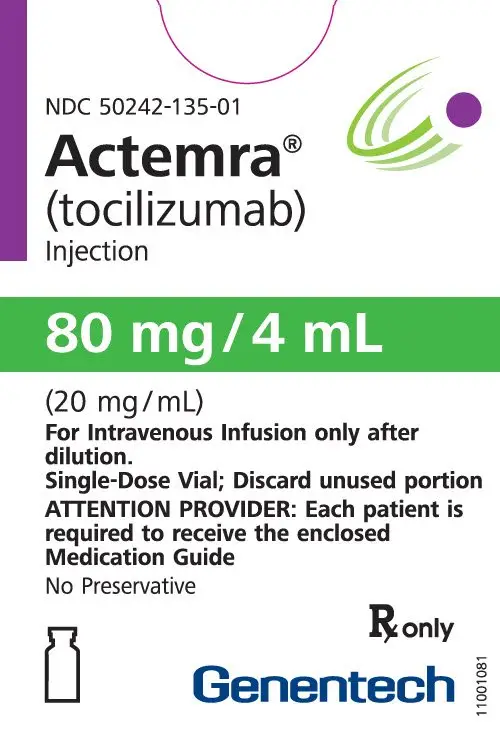
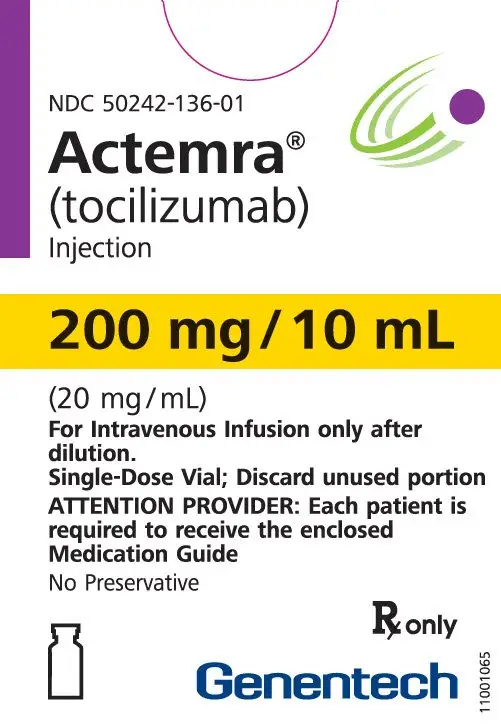
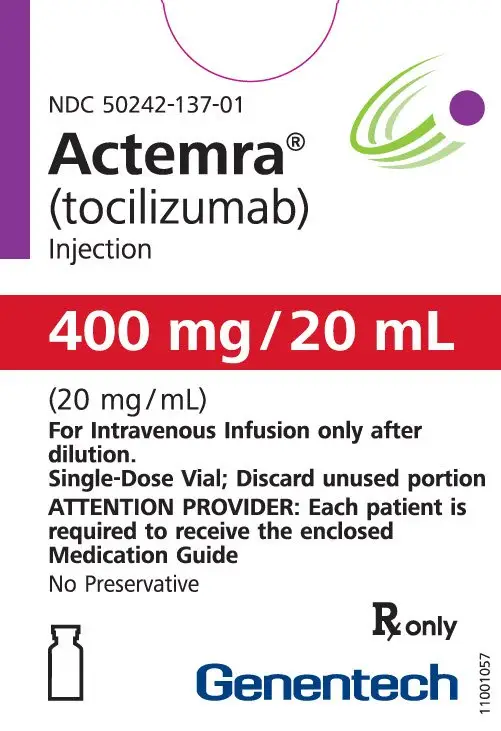
Dosage Forms and Strengths
Intravenous Infusion
Injection: 80 mg/4 mL (20 mg/mL), 200 mg/10 mL (20 mg/mL), 400 mg/20 mL (20 mg/mL) in single-dose vials for further dilution prior to intravenous infusion (3)
Subcutaneous Injection
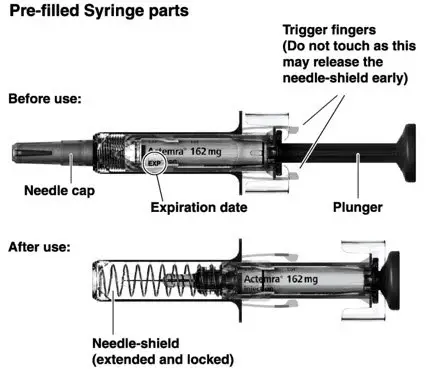
Injection: 162 mg/0.9 mL in a single-dose prefilled syringe or single-dose prefilled ACTPen® autoinjector (3)
Contraindications
- ACTEMRA is contraindicated in patients with known hypersensitivity to ACTEMRA. (4)
Warnings and Precautions
- Serious Infections – do not administer ACTEMRA during an active infection, including localized infections. If a serious infection develops, interrupt ACTEMRA until the infection is controlled. (5.1)
- Gastrointestinal (GI) perforation—use with caution in patients who may be at increased risk. (5.2)
- Hepatotoxicity- Monitor patients for signs and symptoms of hepatic injury. Modify or discontinue ACTEMRA if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop. (2.10, 5.3)
- Laboratory monitoring—recommended due to potential consequences of treatment-related changes in neutrophils, platelets, lipids, and liver function tests. (2.10, 5.4)
- Hypersensitivity reactions, including anaphylaxis and death have occurred. (5.6)
- Live vaccines—Avoid use with ACTEMRA. (5.9, 7.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence of at least 5%): upper respiratory tract infections, nasopharyngitis, headache, hypertension, increased ALT, injection site reactions. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Genentech at 1-888-835-2555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Lactation: Discontinue drug or nursing taking into consideration importance of drug to mother. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2022
Full Prescribing Information
WARNING: RISK OF SERIOUS INFECTIONS
Patients treated with ACTEMRA are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1), Adverse Reactions (6.1)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
If a serious infection develops, interrupt ACTEMRA until the infection is controlled.
Reported infections include:
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients, except those with COVID-19, should be tested for latent tuberculosis before ACTEMRA use and during therapy. Treatment for latent infection should be initiated prior to ACTEMRA use.
- Invasive fungal infections, including candidiasis, aspergillosis, and pneumocystis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
- Bacterial, viral and other infections due to opportunistic pathogens.
The risks and benefits of treatment with ACTEMRA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with ACTEMRA, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see Warnings and Precautions (5.1)].
1. Indications and Usage for Actemra
1.1 Rheumatoid Arthritis (RA)
ACTEMRA® (tocilizumab) is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs).
1.2 Giant Cell Arteritis (GCA)
ACTEMRA® (tocilizumab) is indicated for the treatment of giant cell arteritis (GCA) in adult patients.
1.3 Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD)
ACTEMRA® (tocilizumab) is indicated for slowing the rate of decline in pulmonary function in adult patients with systemic sclerosis-associated interstitial lung disease.
1.4 Polyarticular Juvenile Idiopathic Arthritis (PJIA)
ACTEMRA® (tocilizumab) is indicated for the treatment of active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older.
1.5 Systemic Juvenile Idiopathic Arthritis (SJIA)
ACTEMRA® (tocilizumab) is indicated for the treatment of active systemic juvenile idiopathic arthritis in patients 2 years of age and older.
1.6 Cytokine Release Syndrome (CRS)
ACTEMRA® (tocilizumab) is indicated for the treatment of chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome in adults and pediatric patients 2 years of age and older.
1.7 Coronavirus Disease 2019 (COVID-19)
ACTEMRA® (tocilizumab) is indicated for the treatment of coronavirus disease 2019 (COVID-19) in hospitalized adult patients who are receiving systemic corticosteroids and require supplemental oxygen, non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
2. Actemra Dosage and Administration
2.2 Recommended Dosage for Rheumatoid Arthritis
ACTEMRA may be used as monotherapy or concomitantly with methotrexate or other non-biologic DMARDs as an intravenous infusion or as a subcutaneous injection.
2.4 Recommended Dosage for Systemic Sclerosis-Associated Interstitial Lung Disease
The recommended dose of ACTEMRA for adult patients with SSc-ILD is 162 mg given once every week as a subcutaneous injection.
- Interruption of dosing may be needed for management of dose-related laboratory abnormalities including elevated liver enzymes, neutropenia, and thrombocytopenia [see Dosage and Administration (2.11)].
- Subcutaneous administration with the prefilled ACTPen® autoinjector has not been studied in SSc-ILD.
- Intravenous administration is not approved for SSc-ILD.
2.5 Recommended Dosage for Polyarticular Juvenile Idiopathic Arthritis
ACTEMRA may be used as an intravenous infusion or as a subcutaneous injection alone or in combination with methotrexate. Do not change dose based solely on a single visit body weight measurement, as weight may fluctuate.
2.6 Recommended Dosage for Systemic Juvenile Idiopathic Arthritis
ACTEMRA may be used as an intravenous infusion or as a subcutaneous injection alone or in combination with methotrexate. Do not change a dose based solely on a single visit body weight measurement, as weight may fluctuate.
2.7 Recommended Dosage for Cytokine Release Syndrome (CRS)
Use only the intravenous route for treatment of CRS. The recommended dose of ACTEMRA for treatment of CRS given as a 60-minute intravenous infusion is:
| Recommended Intravenous CRS Dosage | |
|---|---|
| Patients less than 30 kg weight | 12 mg per kg |
| Patients at or above 30 kg weight | 8 mg per kg |
| Alone or in combination with corticosteroids | |
- If no clinical improvement in the signs and symptoms of CRS occurs after the first dose, up to 3 additional doses of ACTEMRA may be administered. The interval between consecutive doses should be at least 8 hours.
- Doses exceeding 800 mg per infusion are not recommended in CRS patients.
- Subcutaneous administration is not approved for CRS.
2.8 Coronavirus Disease 2019 (COVID-19)
Administer ACTEMRA by intravenous infusion only.
The recommended dosage of ACTEMRA for treatment of adult patients with COVID-19 is 8 mg per kg administered as a single 60-minute intravenous infusion. If clinical signs or symptoms worsen or do not improve after the first dose, one additional infusion of ACTEMRA may be administered at least 8 hours after the initial infusion.
- Doses exceeding 800 mg per infusion are not recommended in patients with COVID-19.
- Subcutaneous administration is not approved for COVID-19.
2.9 Preparation and Administration Instructions for Intravenous Infusion
ACTEMRA for intravenous infusion should be diluted by a healthcare professional using aseptic technique as follows:
- Use a sterile needle and syringe to prepare ACTEMRA.
- Patients less than 30 kg: use a 50 mL infusion bag or bottle of 0.9% or 0.45% Sodium Chloride Injection, USP, and then follow steps 1 and 2 below.
- Patients at or above 30 kg weight: use a 100 mL infusion bag or bottle, and then follow steps 1 and 2 below.
- Step 1. Withdraw a volume of 0.9% or 0.45% Sodium Chloride Injection, USP, equal to the volume of the ACTEMRA injection required for the patient's dose from the infusion bag or bottle [see Dosage and Administration (2.2, 2.5, 2.6, 2.7)].
| For Intravenous Use: Volume of ACTEMRA Injection per kg of Body Weight | ||
|---|---|---|
| Dosage | Indication | Volume of ACTEMRA injection per kg of body weight |
| 4 mg/kg | Adult RA | 0.2 mL/kg |
| 6 mg/kg | Adult GCA | 0.3 mL/kg |
| 8 mg/kg | Adult RA Adult COVID-19 SJIA, PJIA and CRS (greater than or equal to 30 kg of body weight) | 0.4 mL/kg |
| 10 mg/kg | PJIA (less than 30 kg of body weight) | 0.5 mL/kg |
| 12 mg/kg | SJIA and CRS (less than 30 kg of body weight) | 0.6 mL/kg |
- Step 2. Withdraw the amount of ACTEMRA for intravenous infusion from the vial(s) and add slowly into the 0.9% or 0.45% Sodium Chloride Injection, USP infusion bag or bottle. To mix the solution, gently invert the bag to avoid foaming.
- The fully diluted ACTEMRA solutions for infusion using 0.9% Sodium Chloride Injection, USP may be stored at 36°F to 46°F (2°C to 8°C) or room temperature for up to 24 hours and should be protected from light.
- The fully diluted ACTEMRA solutions for infusion using 0.45% Sodium Chloride Injection, USP may be stored at 36°F to 46°F (2°C to 8°C) for up to 24 hours or room temperature for up to 4 hours and should be protected from light.
- ACTEMRA solutions do not contain preservatives; therefore, unused product remaining in the vials should not be used.
- Allow the fully diluted ACTEMRA solution to reach room temperature prior to infusion.
- The infusion should be administered over 60 minutes, and must be administered with an infusion set. Do not administer as an intravenous push or bolus.
- ACTEMRA should not be infused concomitantly in the same intravenous line with other drugs. No physical or biochemical compatibility studies have been conducted to evaluate the co-administration of ACTEMRA with other drugs.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulates and discolorations are noted, the product should not be used.
- Fully diluted ACTEMRA solutions are compatible with polypropylene, polyethylene and polyvinyl chloride infusion bags and polypropylene, polyethylene and glass infusion bottles.
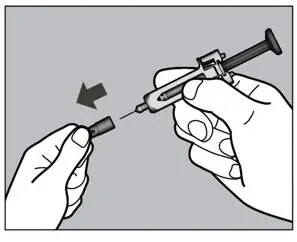
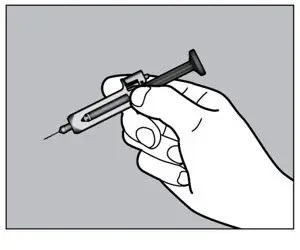
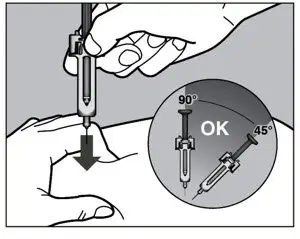
2.10 Preparation and Administration Instructions for Subcutaneous Injection
- ACTEMRA for subcutaneous injection is not intended for intravenous drip infusion.
- Assess suitability of patient for subcutaneous home use and instruct patients to inform a healthcare professional before administering the next dose if they experience any symptoms of allergic reaction. Patients should seek immediate medical attention if they develop symptoms of serious allergic reactions. ACTEMRA subcutaneous injection is intended for use under the guidance of a healthcare practitioner. After proper training in subcutaneous injection technique, a patient may self-inject ACTEMRA or the patient's caregiver may administer ACTEMRA if a healthcare practitioner determines that it is appropriate. PJIA and SJIA patients may self-inject with the ACTEMRA prefilled syringe or ACTPen® autoinjector, or the patient's caregiver may administer ACTEMRA if both the healthcare practitioner and the parent/legal guardian determines it is appropriate [see Use in Specific Populations (8.4)]. Patients, or patient caregivers, should be instructed to follow the directions provided in the Instructions for Use (IFU) for additional details on medication administration.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use ACTEMRA prefilled syringes (PFS) or prefilled ACTPen® autoinjectors exhibiting particulate matter, cloudiness, or discoloration. ACTEMRA for subcutaneous administration should be clear and colorless to pale yellow. Do not use if any part of the PFS or ACTPen® autoinjector appears to be damaged.
- Patients using ACTEMRA for subcutaneous administration should be instructed to inject the full amount in the syringe (0.9 mL) or full amount in the ACTPen® autoinjector (0.9 mL), which provides 162 mg of ACTEMRA, according to the directions provided in the IFU.
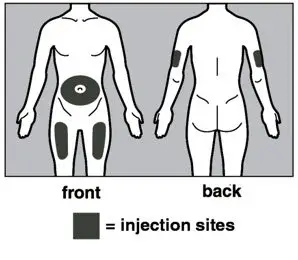
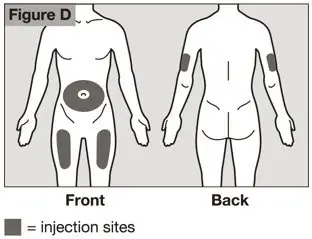
- Injection sites should be rotated with each injection and should never be given into moles, scars, or areas where the skin is tender, bruised, red, hard, or not intact.
4. Contraindications
ACTEMRA is contraindicated in patients with known hypersensitivity to ACTEMRA [see Warnings and Precautions (5.6)].
5. Warnings and Precautions
5.1 Serious Infections
Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, protozoal, or other opportunistic pathogens have been reported in patients receiving immunosuppressive agents including ACTEMRA. The most common serious infections included pneumonia, urinary tract infection, cellulitis, herpes zoster, gastroenteritis, diverticulitis, sepsis and bacterial arthritis [see Adverse Reactions (6.1)]. Among opportunistic infections, tuberculosis, cryptococcus, aspergillosis, candidiasis, and pneumocystosis were reported with ACTEMRA. Other serious infections, not reported in clinical studies, may also occur (e.g., histoplasmosis, coccidioidomycosis, listeriosis). Patients have presented with disseminated rather than localized disease, and were often taking concomitant immunosuppressants such as methotrexate or corticosteroids which in addition to rheumatoid arthritis may predispose them to infections.
Do not administer ACTEMRA in patients with an active infection, including localized infections. The risks and benefits of treatment should be considered prior to initiating ACTEMRA in patients:
- with chronic or recurrent infection;
- who have been exposed to tuberculosis;
- with a history of serious or an opportunistic infection;
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or
- with underlying conditions that may predispose them to infection.
Closely monitor patients for the development of signs and symptoms of infection during and after treatment with ACTEMRA, as signs and symptoms of acute inflammation may be lessened due to suppression of the acute phase reactants [see Dosage and Administration (2.8), Adverse Reactions (6.1), and Patient Counseling Information (17)].
Hold ACTEMRA if a patient develops a serious infection, an opportunistic infection, or sepsis. A patient who develops a new infection during treatment with ACTEMRA should undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, initiate appropriate antimicrobial therapy, and closely monitor the patient.
5.2 Gastrointestinal Perforations
Events of gastrointestinal perforation have been reported in clinical trials, primarily as complications of diverticulitis in patients treated with ACTEMRA. Use ACTEMRA with caution in patients who may be at increased risk for gastrointestinal perforation. Promptly evaluate patients presenting with new onset abdominal symptoms for early identification of gastrointestinal perforation [see Adverse Reactions (6.1)].
5.3 Hepatotoxicity
Serious cases of hepatic injury have been observed in patients taking intravenous or subcutaneous ACTEMRA. Some of these cases have resulted in liver transplant or death. Time to onset for cases ranged from months to years after treatment initiation with tocilizumab. While most cases presented with marked elevations of transaminases (> 5 times ULN), some cases presented with signs or symptoms of liver dysfunction and only mildly elevated transaminases.
During randomized controlled studies, treatment with ACTEMRA was associated with a higher incidence of transaminase elevations [see Adverse Reactions (6.1, 6.2, 6.6, 6.8)]. Increased frequency and magnitude of these elevations was observed when potentially hepatotoxic drugs (e.g., MTX) were used in combination with ACTEMRA.
For RA, GCA and SSc-ILD patients, obtain a liver test panel (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and total bilirubin) before initiating ACTEMRA, every 4 to 8 weeks after start of therapy for the first 6 months of treatment and every 3 months thereafter. It is not recommended to initiate ACTEMRA treatment in RA, GCA or SSc-ILD patients with elevated transaminases ALT or AST greater than 1.5× ULN. In patients who develop elevated ALT or AST greater than 5× ULN, discontinue ACTEMRA. For recommended modifications based upon increase in transaminases see Dosage and Administration (2.11).
Patients hospitalized with COVID-19 may have elevated ALT or AST levels. Multi-organ failure with involvement of the liver is recognized as a complication of severe COVID-19. The decision to administer ACTEMRA should balance the potential benefit of treating COVID-19 against the potential risks of acute treatment with ACTEMRA. It is not recommended to initiate ACTEMRA treatment in COVID-19 patients with elevated ALT or AST above 10 × ULN. Monitor ALT and AST during treatment.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, such as fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (e.g., ALT greater than three times the upper limit of the reference range, serum total bilirubin greater than two times the upper limit of the reference range), ACTEMRA treatment should be interrupted and investigation done to establish the probable cause. ACTEMRA should only be restarted in patients with another explanation for the liver test abnormalities after normalization of the liver tests.
A similar pattern of liver enzyme elevation is noted with ACTEMRA treatment in the PJIA and SJIA populations. Monitor liver test panel at the time of the second administration and thereafter every 4 to 8 weeks for PJIA and every 2 to 4 weeks for SJIA.
5.4 Changes in Laboratory Parameters
5.5 Immunosuppression
The impact of treatment with ACTEMRA on the development of malignancies is not known but malignancies were observed in clinical studies [see Adverse Reactions (6.1)]. ACTEMRA is an immunosuppressant, and treatment with immunosuppressants may result in an increased risk of malignancies.
5.6 Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including anaphylaxis, have been reported in association with ACTEMRA [see Adverse Reactions (6)] and anaphylactic events with a fatal outcome have been reported with intravenous infusion of ACTEMRA. Anaphylaxis and other hypersensitivity reactions that required treatment discontinuation were reported in 0.1% (3 out of 2644) of patients in the 6-month controlled trials of intravenous ACTEMRA, 0.2% (8 out of 4009) of patients in the intravenous all-exposure RA population, 0.7% (8 out of 1068) in the subcutaneous 6-month controlled RA trials, and in 0.7% (10 out of 1465) of patients in the subcutaneous all-exposure population. In the SJIA controlled trial with intravenous ACTEMRA, 1 out of 112 patients (0.9%) experienced hypersensitivity reactions that required treatment discontinuation. In the PJIA controlled trial with intravenous ACTEMRA, 0 out of 188 patients (0%) in the ACTEMRA all-exposure population experienced hypersensitivity reactions that required treatment discontinuation. Reactions that required treatment discontinuation included generalized erythema, rash, and urticaria. Injection site reactions were categorized separately [see Adverse Reactions (6)].
In the postmarketing setting, events of hypersensitivity reactions, including anaphylaxis and death have occurred in patients treated with a range of doses of intravenous ACTEMRA, with or without concomitant therapies. Events have occurred in patients who received premedication. Hypersensitivity, including anaphylaxis events, have occurred both with and without previous hypersensitivity reactions and as early as the first infusion of ACTEMRA [see Adverse Reactions (6.8)]. ACTEMRA for intravenous use should only be infused by a healthcare professional with appropriate medical support to manage anaphylaxis. For ACTEMRA subcutaneous injection, advise patients to seek immediate medical attention if they experience any symptoms of a hypersensitivity reaction. If anaphylaxis or other hypersensitivity reaction occurs, stop administration of ACTEMRA immediately and discontinue ACTEMRA permanently. Do not administer ACTEMRA to patients with known hypersensitivity to ACTEMRA [see Contraindications (4) and Adverse Reactions (6)].
5.7 Demyelinating Disorders
The impact of treatment with ACTEMRA on demyelinating disorders is not known, but multiple sclerosis and chronic inflammatory demyelinating polyneuropathy were reported rarely in RA clinical studies. Monitor patients for signs and symptoms potentially indicative of demyelinating disorders. Prescribers should exercise caution in considering the use of ACTEMRA in patients with preexisting or recent onset demyelinating disorders.
5.8 Active Hepatic Disease and Hepatic Impairment
Treatment with ACTEMRA is not recommended in patients with active hepatic disease or hepatic impairment [see Adverse Reactions (6.1), Use in Specific Populations (8.6)].
5.9 Vaccinations
Avoid use of live vaccines concurrently with ACTEMRA as clinical safety has not been established. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving ACTEMRA.
No data are available on the effectiveness of vaccination in patients receiving ACTEMRA. Because IL-6 inhibition may interfere with the normal immune response to new antigens, it is recommended that all patients, particularly pediatric or elderly patients, if possible, be brought up to date with all immunizations in agreement with current immunization guidelines prior to initiating ACTEMRA therapy. The interval between live vaccinations and initiation of ACTEMRA therapy should be in accordance with current vaccination guidelines regarding immunosuppressive agents.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described elsewhere in labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Gastrointestinal Perforations [see Warnings and Precautions (5.2)]
- Laboratory Parameters [see Warnings and Precautions (5.4)]
- Immunosuppression [see Warnings and Precautions (5.5)]
- Hypersensitivity Reactions, Including Anaphylaxis [see Warnings and Precautions (5.6)]
- Demyelinating Disorders [see Warnings and Precautions (5.7)]
- Active Hepatic Disease and Hepatic Impairment [see Warnings and Precautions (5.8)]
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not predict the rates observed in a broader patient population in clinical practice.
6.1 Clinical Trials Experience in Rheumatoid Arthritis Patients Treated with Intravenous ACTEMRA (ACTEMRA-IV)
The ACTEMRA-IV data in rheumatoid arthritis (RA) includes 5 double-blind, controlled, multicenter studies. In these studies, patients received doses of ACTEMRA-IV 8 mg per kg monotherapy (288 patients), ACTEMRA-IV 8 mg per kg in combination with DMARDs (including methotrexate) (1582 patients), or ACTEMRA-IV 4 mg per kg in combination with methotrexate (774 patients).
The all exposure population includes all patients in registration studies who received at least one dose of ACTEMRA-IV. Of the 4009 patients in this population, 3577 received treatment for at least 6 months, 3309 for at least one year; 2954 received treatment for at least 2 years and 2189 for 3 years.
All patients in these studies had moderately to severely active rheumatoid arthritis. The study population had a mean age of 52 years, 82% were female and 74% were Caucasian.
The most common serious adverse reactions were serious infections [see Warnings and Precautions (5.1)]. The most commonly reported adverse reactions in controlled studies up to 24 weeks (occurring in at least 5% of patients treated with ACTEMRA-IV monotherapy or in combination with DMARDs) were upper respiratory tract infections, nasopharyngitis, headache, hypertension and increased ALT.
The proportion of patients who discontinued treatment due to any adverse reactions during the double-blind, placebo-controlled studies was 5% for patients taking ACTEMRA-IV and 3% for placebo-treated patients. The most common adverse reactions that required discontinuation of ACTEMRA-IV were increased hepatic transaminase values (per protocol requirement) and serious infections.
Laboratory Abnormalities
Elevated Liver Enzymes
Liver enzyme abnormalities are summarized in Table 1. In patients experiencing liver enzyme elevation, modification of treatment regimen, such as reduction in the dose of concomitant DMARD, interruption of ACTEMRA-IV, or reduction in ACTEMRA-IV dose, resulted in decrease or normalization of liver enzymes [see Dosage and Administration (2.11)]. These elevations were not associated with clinically relevant increases in direct bilirubin, nor were they associated with clinical evidence of hepatitis or hepatic insufficiency [see Warnings and Precautions (5.3, 5.4)].
| ACTEMRA 8 mg per kg MONOTHERAPY | Methotrexate | ACTEMRA 4 mg per kg + DMARDs | ACTEMRA 8 mg per kg + DMARDs | Placebo + DMARDs | |
|---|---|---|---|---|---|
| N = 288 (%) | N = 284 (%) | N = 774 (%) | N = 1582 (%) | N = 1170 (%) |
|
| ULN = Upper Limit of Normal | |||||
|
|||||
| AST (U/L) | |||||
| > ULN to 3× ULN | 22 | 26 | 34 | 41 | 17 |
| > 3× ULN to 5× ULN | 0.3 | 2 | 1 | 2 | 0.3 |
| > 5× ULN | 0.7 | 0.4 | 0.1 | 0.2 | < 0.1 |
| ALT (U/L) | |||||
| > ULN to 3× ULN | 36 | 33 | 45 | 48 | 23 |
| > 3× ULN to 5× ULN | 1 | 4 | 5 | 5 | 1 |
| > 5× ULN | 0.7 | 1 | 1.3 | 1.5 | 0.3 |
In the all-exposure population, the elevations in ALT and AST remained consistent with what was seen in the 24 week, controlled clinical trials.
In Study WA25204, of the 1538 patients with moderate to severe RA [see Clinical Studies (14.1)] and treated with tocilizumab, elevations in ALT or AST >3 × ULN occurred in 5.3% and 2.2% patients, respectively. One serious event of drug induced hepatitis with hyperbilirubinemia was reported in association with tocilizumab.
Other Adverse Reactions
Adverse reactions occurring in 2% or more of patients on 4 or 8 mg per kg ACTEMRA-IV plus DMARD and at least 1% greater than that observed in patients on placebo plus DMARD are summarized in Table 2.
| 24 Week Phase 3 Controlled Study Population | |||||
|---|---|---|---|---|---|
| ACTEMRA 8 mg per kg MONOTHERAPY | Methotrexate | ACTEMRA 4 mg per kg + DMARDs | ACTEMRA 8 mg per kg + DMARDs | Placebo + DMARDs | |
| Preferred Term | N = 288 (%) | N = 284 (%) | N = 774 (%) | N = 1582 (%) | N = 1170 (%) |
| Upper Respiratory Tract Infection | 7 | 5 | 6 | 8 | 6 |
| Nasopharyngitis | 7 | 6 | 4 | 6 | 4 |
| Headache | 7 | 2 | 6 | 5 | 3 |
| Hypertension | 6 | 2 | 4 | 4 | 3 |
| ALT increased | 6 | 4 | 3 | 3 | 1 |
| Dizziness | 3 | 1 | 2 | 3 | 2 |
| Bronchitis | 3 | 2 | 4 | 3 | 3 |
| Rash | 2 | 1 | 4 | 3 | 1 |
| Mouth Ulceration | 2 | 2 | 1 | 2 | 1 |
| Abdominal Pain Upper | 2 | 2 | 3 | 3 | 2 |
| Gastritis | 1 | 2 | 1 | 2 | 1 |
| Transaminase increased | 1 | 5 | 2 | 2 | 1 |
Other infrequent and medically relevant adverse reactions occurring at an incidence less than 2% in rheumatoid arthritis patients treated with ACTEMRA-IV in controlled trials were:
Infections and Infestations: oral herpes simplex
Gastrointestinal disorders: stomatitis, gastric ulcer
Investigations: weight increased, total bilirubin increased
Blood and lymphatic system disorders: leukopenia
General disorders and administration site conditions: edema peripheral
Respiratory, thoracic, and mediastinal disorders: dyspnea, cough
Eye disorders: conjunctivitis
Renal disorders: nephrolithiasis
Endocrine disorders: hypothyroidism
6.2 Clinical Trials Experience in Rheumatoid Arthritis Patients Treated with Subcutaneous ACTEMRA (ACTEMRA-SC)
The ACTEMRA-SC data in rheumatoid arthritis (RA) includes 2 double-blind, controlled, multicenter studies. Study SC-I was a non-inferiority study that compared the efficacy and safety of tocilizumab 162 mg administered every week subcutaneously and 8 mg/kg intravenously every four weeks in 1262 adult subjects with rheumatoid arthritis. Study SC-II was a placebo controlled superiority study that evaluated the safety and efficacy of tocilizumab 162 mg administered every other week subcutaneously or placebo in 656 patients. All patients in both studies received background non-biologic DMARDs.
The safety observed for ACTEMRA-SC administered subcutaneously was consistent with the known safety profile of intravenous ACTEMRA, with the exception of injection site reactions (ISRs), which were more common with ACTEMRA-SC compared with placebo SC injections (IV arm).
6.3 Clinical Trials Experience in Giant Cell Arteritis Patients Treated with Subcutaneous ACTEMRA (ACTEMRA-SC)
The safety of subcutaneous ACTEMRA (tocilizumab) has been studied in one Phase III study (WA28119) with 251 GCA patients. The total patient years duration in the ACTEMRA-SC GCA all exposure population was 138.5 patient years during the 12-month double blind, placebo-controlled phase of the study. The overall safety profile observed in the ACTEMRA-SC treatment groups was generally consistent with the known safety profile of ACTEMRA. There was an overall higher incidence of infections in GCA patients relative to RA patients. The rate of infection/serious infection events was 200.2/9.7 events per 100 patient years in the ACTEMRA-SC weekly group and 160.2/4.4 events per 100 patient years in the ACTEMRA-SC every other week group as compared to 156.0/4.2 events per 100 patient years in the placebo + 26 week prednisone taper and 210.2/12.5 events per 100 patient years in the placebo + 52 week taper groups.
6.4 Clinical Trials Experience in Giant Cell Arteritis Patients Treated With Intravenous ACTEMRA (ACTEMRA-IV)
The safety of ACTEMRA-IV was studied in an open label PK-PD and safety study in 24 patients with GCA who were in remission on ACTEMRA-IV at time of enrollment. Patients received ACTEMRA 7 mg/kg every 4 weeks for 20 weeks, followed by 6 mg/kg every 4 weeks for 20 weeks. The total patient years exposure to treatment was 17.5 years. The overall safety profile observed for ACTEMRA administered intravenously in GCA patients was consistent with the known safety profile of ACTEMRA.
6.5 Clinical Trials Experience in Systemic Sclerosis-Associated Interstitial Lung Disease Patients Treated with Subcutaneous ACTEMRA (ACTEMRA-SC)
The safety of subcutaneous ACTEMRA was evaluated in two double-blind, placebo-controlled, multicenter studies (WA29767 and WA27788). In the Phase 3 Study WA29767, 212 patients with SSc were randomized to tocilizumab 162 mg administered every week subcutaneously or placebo for 48 weeks, followed by open-label tocilizumab 162 mg administered subcutaneously every week for another 48 weeks. In the Phase 2/3 Study WA27788, 87 patients were randomized to tocilizumab 162 mg administered every week subcutaneously or placebo for 48 weeks, followed by open-label tocilizumab 162 mg administered subcutaneously every week for another 48 weeks.
The safety profile for ACTEMRA through week 48 in WA29767 was comparable for SSc-ILD and SSc patients overall, and in both studies was consistent with the known safety profile of ACTEMRA.
6.6 Clinical Trials Experience in Polyarticular Juvenile Idiopathic Arthritis Patients Treated With Intravenous ACTEMRA (ACTEMRA-IV)
The safety of ACTEMRA-IV was studied in 188 pediatric patients 2 to 17 years of age with PJIA who had an inadequate clinical response or were intolerant to methotrexate. The total patient exposure in the ACTEMRA-IV all exposure population (defined as patients who received at least one dose of ACTEMRA-IV) was 184.4 patient years. At baseline, approximately half of the patients were taking oral corticosteroids and almost 80% were taking methotrexate. In general, the types of adverse drug reactions in patients with PJIA were consistent with those seen in RA and SJIA patients [see Adverse Reactions (6.1 and 6.8)].
6.7 Clinical Trials Experience in Polyarticular Juvenile Idiopathic Arthritis Patients Treated With Subcutaneous ACTEMRA (ACTEMRA-SC)
The safety of ACTEMRA-SC was studied in 52 pediatric patients 1 to 17 years of age with PJIA who had an inadequate clinical response or were intolerant to methotrexate. The total patient exposure in the PJIA ACTEMRA-SC population (defined as patients who received at least one dose of ACTEMRA-SC and accounting for treatment discontinuation) was 49.5 patient years. In general, the safety observed for ACTEMRA administered subcutaneously was consistent with the known safety profile of intravenous ACTEMRA, with the exception of injection site reactions (ISRs), and neutropenia.
6.8 Clinical Trials Experience in Systemic Juvenile Idiopathic Arthritis Patients Treated with Intravenous ACTEMRA (ACTEMRA-IV)
The data described below reflect exposure to ACTEMRA-IV in one randomized, double-blind, placebo-controlled trial of 112 pediatric patients with SJIA 2 to 17 years of age who had an inadequate clinical response to nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids due to toxicity or lack of efficacy. At baseline, approximately half of the patients were taking 0.3 mg/kg/day corticosteroids or more, and almost 70% were taking methotrexate. The trial included a 12 week controlled phase followed by an open-label extension. In the 12 week double-blind, controlled portion of the clinical study 75 patients received treatment with ACTEMRA-IV (8 or 12 mg per kg based upon body weight). After 12 weeks or at the time of escape, due to disease worsening, patients were treated with ACTEMRA-IV in the open-label extension phase.
The most common adverse events (at least 5%) seen in ACTEMRA-IV treated patients in the 12 week controlled portion of the study were: upper respiratory tract infection, headache, nasopharyngitis and diarrhea.
6.9 Clinical Trials Experience in Systemic Juvenile Idiopathic Arthritis Patients Treated with Subcutaneous ACTEMRA (ACTEMRA-SC)
The safety profile of ACTEMRA-SC was studied in 51 pediatric patients 1 to 17 years of age with SJIA who had an inadequate clinical response to NSAIDs and corticosteroids. In general, the safety observed for ACTEMRA administered subcutaneously was consistent with the known safety profile of intravenous ACTEMRA, with the exception of ISRs where a higher frequency was observed in ACTEMRA-SC treated SJIA patients compared to PJIA patients and adult RA or GCA patients [see Adverse Reactions (6.2, 6.3 and 6.7)].
6.10 Clinical Trials Experience in Patients with Cytokine Release Syndrome Treated with Intravenous ACTEMRA (ACTEMRA-IV)
In a retrospective analysis of pooled outcome data from multiple clinical trials 45 patients were treated with tocilizumab 8 mg/kg (12 mg/kg for patients less than 30 kg) with or without additional high-dose corticosteroids for severe or life-threatening CAR T-cell-induced CRS. A median of 1 dose of tocilizumab (range, 1-4 doses) was administered. No adverse reactions related to tocilizumab were reported [see Clinical Studies (14.10)].
6.11 Clinical Trials Experience in COVID-19 Patients Treated with Intravenous ACTEMRA (ACTEMRA-IV)
The safety of ACTEMRA in hospitalized COVID-19 patients was evaluated in a pooled safety population that includes patients enrolled in EMPACTA, COVACTA, AND REMDACTA. The analysis of adverse reactions included a total of 974 patients exposed to ACTEMRA. Patients received a single, 60-minute infusion of intravenous ACTEMRA 8 mg/kg (maximum dose of 800 mg). If clinical signs or symptoms worsened or did not improve, one additional dose of ACTEMRA 8 mg/kg could be administered between 8- 24 hours after the initial dose.
Adverse reactions summarized in Table 3 occurred in at least 3% of ACTEMRA-treated patients and more commonly than in patients on placebo in the pooled safety population.
| Adverse Reaction | ACTEMRA 8 mg per kg | Placebo |
|---|---|---|
| N = 974 (%) | N = 483 (%) |
|
|
||
| Hepatic Transaminases increased | 10% | 8% |
| Constipation | 9 % | 8% |
| Urinary tract infection | 5% | 4% |
| Hypertension | 4% | 1% |
| Hypokalaemia | 4% | 3% |
| Anxiety | 4% | 2% |
| Diarrhea | 4% | 2% |
| Insomnia | 4% | 3% |
| Nausea | 3% | 2% |
In the pooled safety population, the rates of infection/serious infection events were 30%/19% in patients receiving ACTEMRA versus 32%/23% receiving placebo.
6.12 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ACTEMRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Fatal anaphylaxis [see Warnings and Precautions (5.6)]
- Stevens-Johnson Syndrome
- Pancreatitis
- Drug-induced liver injury, Hepatitis, Hepatic failure, Jaundice [see Warnings and Precautions (5.3)]
7. Drug Interactions
7.1 Concomitant Drugs for Treatment of Adult Indications
In RA patients, population pharmacokinetic analyses did not detect any effect of methotrexate (MTX), non-steroidal anti-inflammatory drugs or corticosteroids on tocilizumab clearance. Concomitant administration of a single intravenous dose of 10 mg/kg ACTEMRA with 10-25 mg MTX once weekly had no clinically significant effect on MTX exposure. ACTEMRA has not been studied in combination with biological DMARDs such as TNF antagonists [see Dosage and Administration (2.2)].
In GCA patients, no effect of concomitant corticosteroid on tocilizumab exposure was observed.
7.2 Interactions with CYP450 Substrates
Cytochrome P450s in the liver are down-regulated by infection and inflammation stimuli including cytokines such as IL-6. Inhibition of IL-6 signaling in RA patients treated with tocilizumab may restore CYP450 activities to higher levels than those in the absence of tocilizumab leading to increased metabolism of drugs that are CYP450 substrates. In vitro studies showed that tocilizumab has the potential to affect expression of multiple CYP enzymes including CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4. Its effect on CYP2C8 or transporters is unknown. In vivo studies with omeprazole, metabolized by CYP2C19 and CYP3A4, and simvastatin, metabolized by CYP3A4, showed up to a 28% and 57% decrease in exposure one week following a single dose of ACTEMRA, respectively. The effect of tocilizumab on CYP enzymes may be clinically relevant for CYP450 substrates with narrow therapeutic index, where the dose is individually adjusted. Upon initiation or discontinuation of ACTEMRA, in patients being treated with these types of medicinal products, perform therapeutic monitoring of effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) and the individual dose of the medicinal product adjusted as needed. Exercise caution when coadministering ACTEMRA with CYP3A4 substrate drugs where decrease in effectiveness is undesirable, e.g., oral contraceptives, lovastatin, atorvastatin, etc. The effect of tocilizumab on CYP450 enzyme activity may persist for several weeks after stopping therapy [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
ACTEMRA by intravenous use is indicated for the treatment of pediatric patients with:
- Active systemic juvenile idiopathic arthritis in patients 2 years of age and older
- Active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older
- Severe or life-threatening CAR T cell-induced cytokine release syndrome (CRS) in patients 2 years of age and older.
ACTEMRA by subcutaneous use is indicated for the treatment of pediatric patients with:
- Active polyarticular juvenile idiopathic arthritis in patients 2 years of age and older
- Active systemic juvenile idiopathic arthritis in patients 2 years of age and older
The safety and effectiveness of ACTEMRA in pediatric patients with conditions other than PJIA, SJIA or CRS have not been established. The safety and effectiveness in pediatric patients below the age of 2 have not been established in PJIA, SJIA, or CRS.
8.5 Geriatric Use
Of the 2644 patients who received ACTEMRA in Studies I to V [see Clinical Studies (14)], a total of 435 rheumatoid arthritis patients were 65 years of age and older, including 50 patients 75 years and older. Of the 1069 patients who received ACTEMRA-SC in studies SC-I and SC-II there were 295 patients 65 years of age and older, including 41 patients 75 years and older. The frequency of serious infection among ACTEMRA treated subjects 65 years of age and older was higher than those under the age of 65. As there is a higher incidence of infections in the elderly population in general, caution should be used when treating the elderly.
Clinical studies that included ACTEMRA for CRS did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
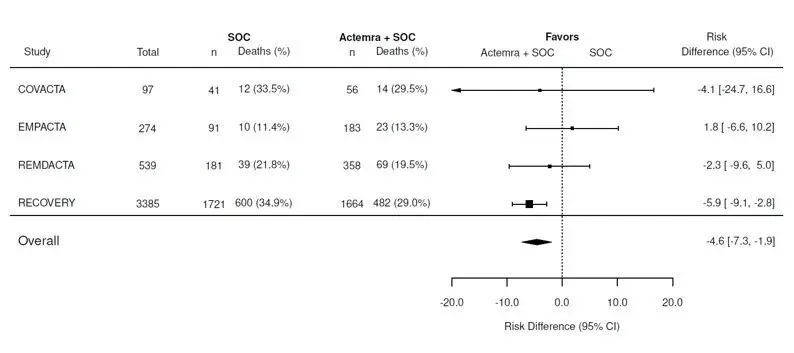
In the EMPACTA, COVACTA, and REMDACTA studies, of the 974 COVID-19 patients in the ACTEMRA arm, 375 (39%) were 65 years of age or older. No overall differences in safety or effectiveness of ACTEMRA were observed between patients 65 years of age and older and those under the age of 65 years of age in these studies [see Adverse Reactions (6.1) and Clinical Studies (14.11)].
In the RECOVERY study, of the 2022 COVID-19 patients in the ACTEMRA arm, 930 (46%) were 65 years of age or older. No overall differences in effectiveness of ACTEMRA were observed between patients 65 years of age and older and those under the age 65 years of age in this study [see Clinical Studies (14.11)].
9. Drug Abuse and Dependence
No studies on the potential for ACTEMRA to cause dependence have been performed. However, there is no evidence from the available data that ACTEMRA treatment results in dependence.
10. Overdosage
There are limited data available on overdoses with ACTEMRA. One case of accidental overdose was reported with intravenous ACTEMRA in which a patient with multiple myeloma received a dose of 40 mg per kg. No adverse drug reactions were observed. No serious adverse drug reactions were observed in healthy volunteers who received single doses of up to 28 mg per kg, although all 5 patients at the highest dose of 28 mg per kg developed dose-limiting neutropenia.
In case of an overdose, it is recommended that the patient be monitored for signs and symptoms of adverse reactions. Patients who develop adverse reactions should receive appropriate symptomatic treatment.
11. Actemra Description
Tocilizumab is a recombinant humanized anti-human interleukin 6 (IL-6) receptor monoclonal antibody of the immunoglobulin IgG1κ (gamma 1, kappa) subclass with a typical H2L2 polypeptide structure. Each light chain and heavy chain consists of 214 and 448 amino acids, respectively. The four polypeptide chains are linked intra- and inter-molecularly by disulfide bonds. ACTEMRA has a molecular weight of approximately 148 kDa. The antibody is produced in mammalian (Chinese hamster ovary) cells.
12. Actemra - Clinical Pharmacology
12.1 Mechanism of Action
Tocilizumab binds to both soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R), and has been shown to inhibit IL-6-mediated signaling through these receptors. IL-6 is a pleiotropic pro-inflammatory cytokine produced by a variety of cell types including T- and B-cells, lymphocytes, monocytes and fibroblasts. IL-6 has been shown to be involved in diverse physiological processes such as T-cell activation, induction of immunoglobulin secretion, initiation of hepatic acute phase protein synthesis, and stimulation of hematopoietic precursor cell proliferation and differentiation. IL-6 is also produced by synovial and endothelial cells leading to local production of IL-6 in joints affected by inflammatory processes such as rheumatoid arthritis.
12.2 Pharmacodynamics
In clinical studies in RA patients with the 4 mg per kg and 8 mg per kg intravenous doses or the 162 mg weekly and every other weekly subcutaneous doses of ACTEMRA, decreases in levels of C-reactive protein (CRP) to within normal ranges were seen as early as week 2. Changes in pharmacodynamic parameters were observed (i.e., decreases in rheumatoid factor, erythrocyte sedimentation rate (ESR), serum amyloid A, fibrinogen and increases in hemoglobin) with doses, however the greatest improvements were observed with 8 mg per kg ACTEMRA. Pharmacodynamic changes were also observed to occur after ACTEMRA administration in GCA, SSc-ILD, PJIA, and SJIA patients (decreases in CRP, ESR, and increases in hemoglobin). The relationship between these pharmacodynamic findings and clinical efficacy is not known.
In healthy subjects administered ACTEMRA in doses from 2 to 28 mg per kg intravenously and 81 to 162 mg subcutaneously, absolute neutrophil counts decreased to the nadir 3 to 5 days following ACTEMRA administration. Thereafter, neutrophils recovered towards baseline in a dose dependent manner. Rheumatoid arthritis and GCA patients demonstrated a similar pattern of absolute neutrophil counts following ACTEMRA administration [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
PK of tocilizumab is characterized by nonlinear elimination which is a combination of linear clearance and Michaelis-Menten elimination. The nonlinear part of tocilizumab elimination leads to an increase in exposure that is more than dose-proportional. The pharmacokinetic parameters of tocilizumab do not change with time. Due to the dependence of total clearance on tocilizumab serum concentrations, the half-life of tocilizumab is also concentration-dependent and varies depending on the serum concentration level. Population pharmacokinetic analyses in any patient population tested so far indicate no relationship between apparent clearance and the presence of anti-drug antibodies.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to establish the carcinogenicity potential of tocilizumab. Literature indicates that the IL-6 pathway can mediate anti-tumor responses by promoting increased immune cell surveillance of the tumor microenvironment. However, available published evidence also supports that IL-6 signaling through the IL-6 receptor may be involved in pathways that lead to tumorigenesis. The malignancy risk in humans from an antibody that disrupts signaling through the IL-6 receptor, such as tocilizumab, is presently unknown.
Fertility and reproductive performance were unaffected in male and female mice that received a murine analogue of tocilizumab administered by the intravenous route at a dose of 50 mg/kg every three days.
14. Clinical Studies
14.1 Rheumatoid Arthritis – Intravenous Administration
The efficacy and safety of intravenously administered ACTEMRA was assessed in five randomized, double-blind, multicenter studies in patients greater than 18 years with active rheumatoid arthritis diagnosed according to American College of Rheumatology (ACR) criteria. Patients had at least 8 tender and 6 swollen joints at baseline. ACTEMRA was given intravenously every 4 weeks as monotherapy (Study I), in combination with methotrexate (MTX) (Studies II and III) or other disease-modifying anti-rheumatic drugs (DMARDs) (Study IV) in patients with an inadequate response to those drugs, or in combination with MTX in patients with an inadequate response to TNF antagonists (Study V).
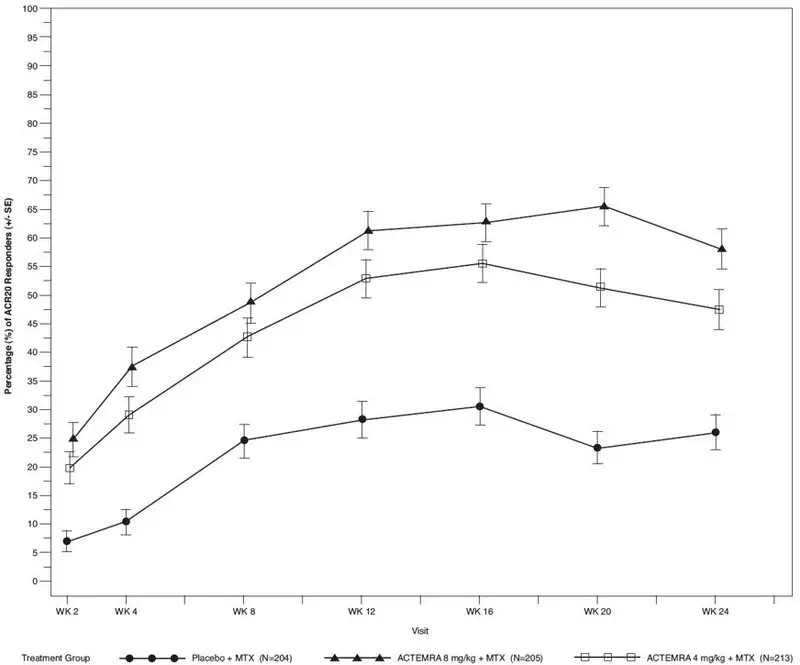
Study I (NCT00109408) evaluated patients with moderate to severe active rheumatoid arthritis who had not been treated with MTX within 24 weeks prior to randomization, or who had not discontinued previous methotrexate treatment as a result of clinically important toxic effects or lack of response. In this study, 67% of patients were MTX-naïve, and over 40% of patients had rheumatoid arthritis less than 2 years. Patients received ACTEMRA 8 mg per kg monotherapy or MTX alone (dose titrated over 8 weeks from 7.5 mg to a maximum of 20 mg weekly). The primary endpoint was the proportion of ACTEMRA patients who achieved an ACR 20 response at Week 24.
Study II (NCT00106535) was a 104-week study with an optional 156-week extension phase that evaluated patients with moderate to severe active rheumatoid arthritis who had an inadequate clinical response to MTX. Patients received ACTEMRA 8 mg per kg, ACTEMRA 4 mg per kg, or placebo every four weeks, in combination with MTX (10 to 25 mg weekly). Upon completion of 52-weeks, patients received open-label treatment with ACTEMRA 8 mg per kg through 104 weeks or they had the option to continue their double-blind treatment if they maintained a greater than 70% improvement in swollen/tender joint count. Two pre-specified interim analyses at week 24 and week 52 were conducted. The primary endpoint at week 24 was the proportion of patients who achieved an ACR 20 response. At weeks 52 and 104, the primary endpoints were change from baseline in modified total Sharp-Genant score and the area under the curve (AUC) of the change from baseline in HAQ-DI score.
Study III (NCT00106548) evaluated patients with moderate to severe active rheumatoid arthritis who had an inadequate clinical response to MTX. Patients received ACTEMRA 8 mg per kg, ACTEMRA 4 mg per kg, or placebo every four weeks, in combination with MTX (10 to 25 mg weekly). The primary endpoint was the proportion of patients who achieved an ACR 20 response at week 24.
Study IV (NCT00106574) evaluated patients who had an inadequate response to their existing therapy, including one or more DMARDs. Patients received ACTEMRA 8 mg per kg or placebo every four weeks, in combination with the stable DMARDs. The primary endpoint was the proportion of patients who achieved an ACR 20 response at week 24.
Study V (NCT00106522) evaluated patients with moderate to severe active rheumatoid arthritis who had an inadequate clinical response or were intolerant to one or more TNF antagonist therapies. The TNF antagonist therapy was discontinued prior to randomization. Patients received ACTEMRA 8 mg per kg, ACTEMRA 4 mg per kg, or placebo every four weeks, in combination with MTX (10 to 25 mg weekly). The primary endpoint was the proportion of patients who achieved an ACR 20 response at week 24.
Clinical Response
The percentages of intravenous ACTEMRA-treated patients achieving ACR 20, 50 and 70 responses are shown in Table 4. In all intravenous studies, patients treated with 8 mg per kg ACTEMRA had higher ACR 20, ACR 50, and ACR 70 response rates versus MTX- or placebo-treated patients at week 24.
During the 24 week controlled portions of Studies I to V, patients treated with ACTEMRA at a dose of 4 mg per kg in patients with inadequate response to DMARDs or TNF antagonist therapy had lower response rates compared to patients treated with ACTEMRA 8 mg per kg.
| Percent of Patients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response Rate | Study I | Study II | Study III | Study IV | Study V | ||||||||
| MTX | ACTEMRA 8 mg per kg | Placebo + MTX | ACTEMRA 4 mg per kg + MTX | ACTEMRA 8 mg per kg + MTX | Placebo + MTX | ACTEMRA 4 mg per kg + MTX | ACTEMRA 8 mg per kg + MTX | Placebo + DMARDs | ACTEMRA 8 mg per kg + DMARDs | Placebo + MTX | ACTEMRA 4 mg per kg + MTX | ACTEMRA 8 mg per kg + MTX | |
| N=284 | N=286 | N=393 | N=399 | N=398 | N=204 | N=213 | N=205 | N=413 | N=803 | N=158 | N=161 | N=170 | |
| (95% CI)* | ( 95% CI)* | (95% CI)* | ( 95% CI)* | (95% CI)* | (95% CI)* | ( 95% CI)* | (95% CI)* | ||||||
|
|||||||||||||
| ACR 20 | |||||||||||||
| Week 24 | 53% | 70% (0.11, 0.27) | 27% | 51% (0.17, 0.29) | 56% (0.23, 0.35) | 27% | 48% (0.15, 0.32) | 59% (0.23, 0.41) | 24% | 61% (0.30, 0.40) | 10% | 30% (0.15, 0.36) | 50% (0.36, 0.56) |
| Week 52 | N/A | N/A | 25% | 47% (0.15, 0.28) | 56% (0.25, 0.38) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| ACR 50 | |||||||||||||
| Week 24 | 34% | 44% (0.04, 0.20) | 10% | 25% (0.09, 0.20) | 32% (0.16, 0.28) | 11% | 32% (0.13, 0.29) | 44% (0.25, 0.41) | 9% | 38% (0.23, 0.33) | 4% | 17% (0.05, 0.25) | 29% (0.21, 0.41) |
| Week 52 | N/A | N/A | 10% | 29% (0.14, 0.25) | 36% (0.21, 0.32) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| ACR 70 | |||||||||||||
| Week 24 | 15% | 28% (0.07, 0.22) | 2% | 11% (0.03, 0.13) | 13% (0.05, 0.15) | 2% | 12% (0.04, 0.18) | 22% (0.12, 0.27) | 3% | 21% (0.13, 0.21) | 1% | 5% (-0.06, 0.14) | 12% (0.03, 0.22) |
| Week 52 | N/A | N/A | 4% | 16% (0.08, 0.17) | 20% (0.12, 0.21) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Major Clinical Responses † | |||||||||||||
| Week 52 | N/A | N/A | 1% | 4% (0.01, 0.06) | 7% (0.03, 0.09) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
In study II, a greater proportion of patients treated with 4 mg per kg and 8 mg per kg ACTEMRA + MTX achieved a low level of disease activity as measured by a DAS 28-ESR less than 2.6 compared with placebo +MTX treated patients at week 52. The proportion of ACTEMRA-treated patients achieving DAS 28-ESR less than 2.6, and the number of residual active joints in these responders in Study II are shown in Table 5.
| Study II | |||
|---|---|---|---|
| Placebo + MTX N = 393 | ACTEMRA 4 mg per kg + MTX N = 399 | ACTEMRA 8 mg per kg + MTX N = 398 |
|
| *n denotes numerator of all the percentage. Denominator is the intent-to-treat population. Not all patients received DAS28 assessments at Week 52. | |||
| DAS28-ESR less than 2.6 | |||
| Proportion of responders at week 52 (n) 95% confidence interval | 3% (12) | 18% (70) 0.10, 0.19 | 32% (127) 0.24, 0.34 |
| Of responders, proportion with 0 active joints (n) | 33% (4) | 27% (19) | 21% (27) |
| Of responders, proportion with 1 active joint (n) | 8% (1) | 19% (13) | 13% (16) |
| Of responders, proportion with 2 active joints (n) | 25% (3) | 13% (9) | 20% (25) |
| Of responders, proportion with 3 or more active joints (n) | 33% (4) | 41% (29) | 47% (59) |
The results of the components of the ACR response criteria for Studies III and V are shown in Table 6. Similar results to Study III were observed in Studies I, II and IV.
| Study III | Study V | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTEMRA 4 mg per kg + MTX | ACTEMRA 8 mg per kg + MTX | Placebo + MTX | ACTEMRA 4 mg per kg + MTX | ACTEMRA 8 mg per kg + MTX | Placebo + MTX | |||||||
| N=213 | N=205 | N=204 | N=161 | N=170 | N=158 | |||||||
| Component (mean) | Baseline | Week 24* | Baseline | Week 24* | Baseline | Week 24 | Baseline | Week 24* | Baseline | Week 24* | Baseline | Week 24 |
|
||||||||||||
| Number of tender joints (0-68) | 33 | 19 -7.0 (-10.0, -4.1) | 32 | 14.5 -9.6 (-12.6, -6.7) | 33 | 25 | 31 | 21 -10.8 (-14.6, -7.1) | 32 | 17 -15.1 (-18.8, -11.4) | 30 | 30 |
| Number of swollen joints (0-66) | 20 | 10 -4.2 (-6.1, -2.3) | 19.5 | 8 -6.2 (-8.1, -4.2) | 21 | 15 | 19.5 | 13 -6.2 (-9.0, -3.5) | 19 | 11 -7.2 (-9.9, -4.5) | 19 | 18 |
| Pain† | 61 | 33 -11.0 (-17.0, -5.0) | 60 | 30 -15.8 (-21.7, -9.9) | 57 | 43 | 63.5 | 43 -12.4 (-22.1, -2.1) | 65 | 33 -23.9 (-33.7, -14.1) | 64 | 48 |
| Patient global assessment† | 66 | 34 -10.9 (-17.1, -4.8) | 65 | 31 -14.9 (-20.9, -8.9) | 64 | 45 | 70 | 46 -10.0 (-20.3, 0.3) | 70 | 36 -17.4 (-27.8, -7.0) | 71 | 51 |
| Physician global assessment† | 64 | 26 -5.6 (-10.5, -0.8) | 64 | 23 -9.0 (-13.8, -4.2) | 64 | 32 | 66.5 | 39 -10.5 (-18.6, -2.5) | 66 | 28 -18.2 (-26.3, -10.0) | 67.5 | 43 |
| Disability index (HAQ)‡ | 1.64 | 1.01 -0.18 (-0.34, -0.02) | 1.55 | 0.96 -0.21 (-0.37, -0.05) | 1.55 | 1.21 | 1.67 | 1.39 -0.25 (-0.42, -0.09) | 1.75 | 1.34 -0.34 (-0.51, -0.17) | 1.70 | 1.58 |
| CRP (mg per dL) | 2.79 | 1.17 -1.30 (-2.0, -0.59) | 2.61 | 0.25 -2.156 (-2.86, -1.46) | 2.36 | 1.89 | 3.11 | 1.77 -1.34 (-2.5, -0.15) | 2.80 | 0.28 -2.52 (-3.72, -1.32) | 3.705 | 3.06 |
The percent of ACR 20 responders by visit for Study III is shown in Figure 1. Similar response curves were observed in studies I, II, IV, and V.
|
| Figure 1 Percent of ACR 20 Responders by Visit for Study III (Inadequate Response to MTX)* |
 |
Radiographic Response
In Study II, structural joint damage was assessed radiographically and expressed as change in total Sharp-Genant score and its components, the erosion score and joint space narrowing score. Radiographs of hands/wrists and forefeet were obtained at baseline, 24 weeks, 52 weeks, and 104 weeks and scored by readers unaware of treatments group and visit number. The results from baseline to week 52 are shown in Table 7. ACTEMRA 4 mg per kg slowed (less than 75% inhibition compared to the control group) and ACTEMRA 8 mg per kg inhibited (at least 75% inhibition compared to the control group) the progression of structural damage compared to placebo plus MTX at week 52.
| Placebo + MTX | ACTEMRA 4 mg per kg + MTX | ACTEMRA 8 mg per kg + MTX | |
|---|---|---|---|
| N=294 | N=343 | N=353 | |
| SD = standard deviation | |||
|
|||
| Week 52* | |||
| Total Sharp-Genant Score, Mean (SD) | 1.17 (3.14) | 0.33 (1.30) | 0.25 (0.98) |
| Adjusted Mean difference†
(95%CI) | -0.83 (-1.13, -0.52) | -0.90 (-1.20, -0.59) |
|
| Erosion Score, Mean (SD) | 0.76 (2.14) | 0.20 (0.83) | 0.15 (0.77) |
| Adjusted Mean difference†
(95%CI) | -0.55 (-0.76, -0.34) | -0.60 (-0.80, -0.39) |
|
| Joint Space Narrowing Score, Mean (SD) | 0.41 (1.71) | 0.13 (0.72) | 0.10 (0.49) |
| Adjusted Mean difference†
(95%CI) | -0.28 (-0.44, -0.11) | -0.30 (-0.46, -0.14) |
|
The mean change from baseline to week 104 in Total Sharp-Genant Score for the ACTEMRA 4 mg per kg groups was 0.47 (SD = 1.47) and for the 8 mg per kg groups was 0.34 (SD = 1.24). By the week 104, most patients in the control (placebo + MTX) group had crossed over to active treatment, and results are therefore not included for comparison. Patients in the active groups may have crossed over to the alternate active dose group, and results are reported per original randomized dose group.
In the placebo group, 66% of patients experienced no radiographic progression (Total Sharp-Genant Score change ≤ 0) at week 52 compared to 78% and 83% in the ACTEMRA 4 mg per kg and 8 mg per kg, respectively. Following 104 weeks of treatment, 75% and 83% of patients initially randomized to ACTEMRA 4 mg per kg and 8 mg per kg, respectively, experienced no progression of structural damage compared to 66% of placebo treated patients.
14.2 Rheumatoid Arthritis – Subcutaneous Administration
The efficacy and safety of subcutaneously administered ACTEMRA was assessed in two double-blind, controlled, multicenter studies in patients with active RA. One study, SC-I (NCT01194414), was a non-inferiority study that compared the efficacy and safety of ACTEMRA 162 mg administered every week subcutaneously to 8 mg per kg intravenously every four weeks. The second study, SC-II (NCT01232569), was a placebo controlled superiority study that evaluated the safety and efficacy of ACTEMRA 162 mg administered every other week subcutaneously to placebo. Both SC-I and SC-II required patients to be >18 years of age with moderate to severe active rheumatoid arthritis diagnosed according to ACR criteria who had at least 4 tender and 4 swollen joints at baseline (SC-I) or at least 8 tender and 6 swollen joints at baseline (SC-II), and an inadequate response to their existing DMARD therapy, where approximately 20% also had a history of inadequate response to at least one TNF inhibitor. All patients in both SC studies received background non-biologic DMARD(s).
In SC-I, 1262 patients were randomized 1:1 to receive ACTEMRA-SC 162 mg every week or intravenous ACTEMRA 8 mg/kg every four weeks in combination with DMARD(s). In SC-II, 656 patients were randomized 2:1 to ACTEMRA-SC 162 mg every other week or placebo, in combination with DMARD(s). The primary endpoint in both studies was the proportion of patients who achieved an ACR20 response at Week 24.
The clinical response to 24 weeks of ACTEMRA-SC therapy is shown in Table 8. In SC-I, the primary outcome measure was ACR20 at Week 24. The pre-specified non-inferiority margin was a treatment difference of 12%. The study demonstrated non-inferiority of ACTEMRA with respect to ACR20 at Week 24; ACR50, ACR70, and DAS28 responses are also shown in Table 8. In SC-II, a greater portion of patients treated with ACTEMRA 162 mg subcutaneously every other week achieved ACR20, ACR50, and ACR70 responses compared to placebo-treated patients (Table 8). Further, a greater proportion of patients treated with ACTEMRA 162 mg subcutaneously every other week achieved a low level of disease activity as measured by a DAS28-ESR less than 2.6 at Week 24 compared to those treated with placebo (Table 8).
| SC-I* | SC-II† | |||
|---|---|---|---|---|
| TCZ SC 162 mg every week + DMARD | TCZ IV 8mg/kg + DMARD | TCZ SC 162 mg every other week + DMARD | Placebo + DMARD | |
| N=558 | N=537 | N=437 | N=219 | |
| TCZ = tocilizumab | ||||
|
||||
| ACR20 | ||||
| Week 24 | 69% | 73.4% | 61% | 32% |
| Weighted difference (95% CI) | -4% (-9.2, 1.2) | 30% (22.0, 37.0) | ||
| ACR50 | ||||
| Week 24 | 47% | 49% | 40% | 12% |
| Weighted difference (95% CI) | -2% (-7.5, 4.0) | 28% (21.5, 34.4) | ||
| ACR70 | ||||
| Week 24 | 24% | 28% | 20% | 5% |
| Weighted difference (95% CI) | -4% (-9.0, 1.3) | 15% (9.8, 19.9) | ||
| Change in DAS28 [Adjusted mean] | ||||
| Week 24 | -3.5 | -3.5 | -3.1 | -1.7 |
| Adjusted mean difference (95% CI) | 0 (-0.2, 0.1) | -1.4 (-1.7; -1.1) | ||
| DAS28 < 2.6 | ||||
| Week 24 | 38.4% | 36.9% | 32.0% | 4.0% |
| Weighted difference (95% CI) | 0.9 (-5.0, 6.8) | 28.6 (22.0, 35.2) | ||
The results of the components of the ACR response criteria and the percent of ACR20 responders by visit for ACTEMRA-SC in Studies SC-I and SC-II were consistent with those observed for ACTEMRA-IV.
14.3 Giant Cell Arteritis – Subcutaneous Administration
The efficacy and safety of subcutaneously administered ACTEMRA was assessed in a single, randomized, double-blind, multicenter study in patients with active GCA. In Study WA28119 (NCT01791153), 251 screened patients with new-onset or relapsing GCA were randomized to one of four treatment arms. Two subcutaneous doses of ACTEMRA (162 mg every week and 162 mg every other week) were compared to two different placebo control groups (pre-specified prednisone-taper regimen over 26 weeks and 52 weeks) randomized 2:1:1:1. The study consisted of a 52-week blinded period, followed by a 104-week open-label extension.
All patients received background glucocorticoid (prednisone) therapy. Each of the ACTEMRA-treated groups and one of the placebo-treated groups followed a pre-specified prednisone-taper regimen with the aim to reach 0 mg by 26 weeks, while the second placebo-treated group followed a pre-specified prednisone-taper regimen with the aim to reach 0 mg by 52 weeks designed to be more in keeping with standard practice.
The primary efficacy endpoint was the proportion of patients achieving sustained remission from Week 12 through Week 52. Sustained remission was defined by a patient attaining a sustained (1) absence of GCA signs and symptoms from Week 12 through Week 52, (2) normalization of erythrocyte sedimentation rate (ESR) (to < 30 mm/hr without an elevation to ≥ 30 mm/hr attributable to GCA) from Week 12 through Week 52, (3) normalization of C-reactive protein (CRP) (to < 1 mg/dL, with an absence of successive elevations to ≥ 1mg/dL) from Week 12 through Week 52, and (4) successful adherence to the prednisone taper defined by not more than 100 mg of excess prednisone from Week 12 through Week 52. ACTEMRA 162 mg weekly and 162 mg every other week + 26 weeks prednisone taper both showed superiority in achieving sustained remission from Week 12 through Week 52 compared with placebo + 26 weeks prednisone taper (Table 9). Both ACTEMRA treatment arms also showed superiority compared to the placebo + 52 weeks prednisone taper (Table 9).
| PBO + 26 weeks prednisone taper N=50 | PBO + 52 weeks prednisone taper N=51 | TCZ 162mg SC QW + 26 weeks prednisone taper N=100 | TCZ 162 mg SC Q2W + 26 weeks prednisone taper N=49 |
|
|---|---|---|---|---|
| Patients not completing the study to week 52 were classified as non-responders in the primary and key secondary analysis: PBO+26: 6 (12.0%), PBO+52: 5 (9.8%), TCZ QW: 15 (15.0%), TCZ Q2W: 9 (18.4%). CRP = C-reactive protein ESR = erythrocyte sedimentation rate PBO = placebo Q2W = every other week dose QW = every week dose TCZ = tocilizumab |
||||
|
||||
| Sustained remission* | ||||
| Responders, n (%) | 7 (14.0%) | 9 (17.6%) | 56 (56.0%) | 26 (53.1%) |
| Unadjusted difference in proportions vs PBO + 26 weeks taper (99.5% CI) | N/A | N/A | 42.0% (18.0, 66.0) | 39.1% (12.5 , 65.7) |
| Unadjusted difference in proportions vs PBO + 52 weeks taper (99.5% CI) | N/A | N/A | 38.4% (14.4, 62.3) | 35.4% (8.6, 62.2) |
| Components of Sustained Remission | ||||
| Sustained absence of GCA signs and symptoms†, n (%) | 20 (40.0%) | 23 (45.1%) | 69 (69.0%) | 28 (57.1%) |
| Sustained ESR<30 mm/hr‡, n (%) | 20 (40.0%) | 22 (43.1%) | 83 (83.0%) | 37 (75.5%) |
| Sustained CRP normalization§, n (%) | 17 (34.0%) | 13 (25.5%) | 72 (72.0%) | 34 (69.4%) |
| Successful prednisone tapering¶, n (%) | 10 (20.0%) | 20 (39.2%) | 60 (60.0%) | 28 (57.1%) |
The estimated annual cumulative prednisone dose was lower in the two ACTEMRA dose groups (medians of 1887 mg and 2207 mg on ACTEMRA QW and Q2W, respectively) relative to the placebo arms (medians of 3804 mg and 3902 mg on placebo + 26 weeks prednisone and placebo + 52 weeks prednisone taper, respectively).
14.5 Systemic Sclerosis-Associated Interstitial Lung Disease– Subcutaneous Administration
The clinical efficacy of ACTEMRA was assessed in a phase 3 multicenter, randomized, double-blind, placebo controlled study in patients with SSc (Study WA29767). A phase 2/3 multicenter, randomized, double-blind, placebo controlled study in patients with SSc (Study WA27788) provided supportive information. Study WA29767 (NCT02453256) enrolled adult patients with SSc defined by the 2013 American College of Rheumatology/European League Against Rheumatism classification criteria for SSc, with onset of disease (first non-Raynaud symptom) of ≤ 5 years, modified Rodnan Skin Score (mRSS) of ≥ 10 and ≤ 35 at screening, elevated inflammatory markers (or platelets), and active disease based on at least one of the following: disease duration ≤ 18 months, increase in mRSS ≥ 3 units over 6 months, involvement of one new body area and an increase in mRSS of ≥ 2 over 6 months, or involvement of two new body areas within the previous 6 months, or presence of at least one tendon friction rub. Study WA27788 (NCT01532869) enrolled adult patients with SSc with onset of disease ≤ 5 years, mRSS of ≥ 15 and ≤ 40 at screening, active disease, and elevated inflammatory markers or platelets. Patients in both studies were not permitted to use biologic agents (such as TNF antagonists), alkylating agents, or cyclophosphamide.
In Study WA29767, 212 patients were randomized in a 1:1 ratio to receive weekly SC injections of 162 mg of ACTEMRA or placebo during the 48-week, double-blinded, placebo controlled period. Rescue treatment was allowed during the treatment period after 16 weeks for >10% percent predicted FVC (ppFVC) decline or after 24 weeks for worsening skin fibrosis. The primary efficacy endpoint was change from baseline at Week 48 in mRSS. Change from baseline in FVC at Week 48 was a key secondary endpoint.
In the overall population of Study WA29767, there was not a statistically significant difference in the mean change from baseline to Week 48 in mRSS (primary endpoint) in patients receiving ACTEMRA compared to placebo (difference: -1.73; 95% CI: -3.78, 0.32). There also was not a statistically significant effect on the primary endpoint of mRSS in Study WA27788.
In the overall population of Study WA29767, patients treated with ACTEMRA, as compared to placebo treated patients, were observed to have less decline from baseline in ppFVC and observed FVC at 48 weeks. FVC results from Study WA27788 were similar.
Of the 212 patients who were randomized in Study WA29767, 68 patients (65%) in the ACTEMRA arm and 68 patients (64%) in the placebo arm had SSc-ILD at baseline, as confirmed by a visual read of high resolution computed tomograph (HRCT) by blinded thoracic radiologists. The mean ppFVC at baseline for patients with SSc-ILD identified by HRCT was 79.6% (median 80.5%). Post-hoc analyses were performed to evaluate results within the subgroups of patients with and without SSc-ILD.
Table 10 shows results from Study WA29767 for the changes from baseline to Week 48 in ppFVC, observed FVC, and mRSS both in the overall population and within subgroups based on SSc-ILD status at baseline. The ppFVC and observed FVC results in the overall population were primarily driven by results in the SSc-ILD subgroup. In the SSc-ILD subgroup, the differences in mean changes from baseline to Week 48 for ACTEMRA, as compared to placebo, were 6.47% and 241 mL for ppFVC and observed FVC, respectively. Figure 2 illustrates the mean change from baseline through Week 48 in observed FVC in patients with SSc-ILD.
The results of the key FVC secondary endpoints from Study WA29767 support a conclusion of effectiveness of ACTEMRA in reducing the rate of progressive loss of lung function in the study population. However, in settings where a trial does not provide evidence of an effect on the primary endpoint, the estimated magnitude of effect on other endpoints should be interpreted with caution, and comparisons to results of other products and studies may be misleading.
| Overall Population | Subgroup Without SSc-ILD* | SSc-ILD Subgroup* | ||||
|---|---|---|---|---|---|---|
| PBO QW | TCZ 162 mg QW | PBO QW | TCZ 162 mg QW | PBO QW | TCZ 162 mg QW | |
| Number of patients | 106 | 104 | 36 | 34 | 68 | 68 |
| PBO=placebo; TCZ=tocilizumab; ppFVC = percent predicted forced vital capacity; LSM=least squares mean; mRSS = modified Rodnan skin score; CI=confidence interval | ||||||
|
||||||
| Change from Baseline in mRSS at Week 48 | ||||||
| LSM | -4.41 | -6.14 | -6.16 | -8.56 | -3.77 | -5.88 |
| Difference in LSM, TCZ-placebo (95% CI) | -1.73 (-3.78, 0.32) | -2.40 (-5.59, 0.79) | -2.11 (-4.89, 0.67) | |||
| Change from Baseline in ppFVC at Week 48 | ||||||
| LSM | -4.58 | -0.38 | -0.82 | -0.32 | -6.40 | 0.07 |
| Difference in LSM, TCZ-placebo (95% CI) | 4.20 (2.00, 6.40) | 0.50 (-2.27, 3.27) | 6.47 (3.43, 9.50) | |||
| Change from Baseline in Observed FVC (mL) at Week 48 | ||||||
| LSM | -190 | -24 | -53 | -11 | -255 | -14 |
| Difference in LSM, TCZ-placebo (95% CI) | 167 (83, 250) | 43 (-60, 145) | 241 (124, 358) | |||
Figure 2 Mean Change from Baseline to Week 48 in Observed Forced Vital Capacity in SSc-ILD Patients from Study WA29767
| PBO = placebo; TCZ = tocilizumab; QW = every week dose |
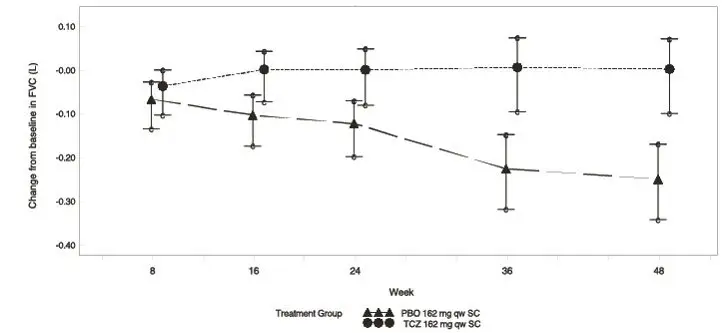 |
14.6 Polyarticular Juvenile Idiopathic Arthritis – Intravenous Administration
The efficacy of ACTEMRA was assessed in a three-part study, WA19977 (NCT00988221), including an open-label extension in children 2 to 17 years of age with active polyarticular juvenile idiopathic arthritis (PJIA), who had an inadequate response to methotrexate or inability to tolerate methotrexate. Patients had at least 6 months of active disease (mean disease duration of 4.2 ± 3.7 years), with at least five joints with active arthritis (swollen or limitation of movement accompanied by pain and/or tenderness) and/or at least 3 active joints having limitation of motion (mean, 20 ± 14 active joints). The patients treated had subtypes of JIA that at disease onset included Rheumatoid Factor Positive or Negative Polyarticular JIA, or Extended Oligoarticular JIA. Treatment with a stable dose of methotrexate was permitted but was not required during the study. Concurrent use of disease modifying antirheumatic drugs (DMARDs), other than methotrexate, or other biologics (e.g., TNF antagonists or T cell costimulation modulator) were not permitted in the study.
Part I consisted of a 16-week active ACTEMRA treatment lead-in period (n=188) followed by Part II, a 24-week randomized double-blind placebo-controlled withdrawal period, followed by Part III, a 64-week open-label period. Eligible patients weighing at or above 30 kg received ACTEMRA at 8 mg/kg intravenously once every four weeks. Patients weighing less than 30 kg were randomized 1:1 to receive either ACTEMRA 8 mg/kg or 10 mg/kg intravenously every four weeks. At the conclusion of the open-label Part I, 91% of patients taking background MTX in addition to tocilizumab and 83% of patients on tocilizumab monotherapy achieved an ACR 30 response at week 16 compared to baseline and entered the blinded withdrawal period (Part II) of the study. The proportions of patients with JIA ACR 50/70 responses in Part I were 84.0%, and 64%, respectively for patients taking background MTX in addition to tocilizumab and 80% and 55% respectively for patients on tocilizumab monotherapy.
In Part II, patients (ITT, n=163) were randomized to ACTEMRA (same dose received in Part I) or placebo in a 1:1 ratio that was stratified by concurrent methotrexate use and concurrent corticosteroid use. Each patient continued in Part II of the study until Week 40 or until the patient satisfied JIA ACR 30 flare criteria (relative to Week 16) and qualified for escape.
The primary endpoint was the proportion of patients with a JIA ACR 30 flare at week 40 relative to week 16. JIA ACR 30 flare was defined as 3 or more of the 6 core outcome variables worsening by at least 30% with no more than 1 of the remaining variables improving by more than 30% relative to Week 16.
ACTEMRA treated patients experienced significantly fewer disease flares compared to placebo-treated patients (26% [21/82] versus 48% [39/81]; adjusted difference in proportions -21%, 95% CI: -35%, -8%).
During the withdrawal phase (Part II), more patients treated with ACTEMRA showed JIA ACR 30/50/70 responses at Week 40 compared to patients withdrawn to placebo.
14.7 Polyarticular Juvenile Idiopathic Arthritis – Subcutaneous Administration
Subcutaneously administered ACTEMRA in pediatric patients with polyarticular juvenile idiopathic arthritis (PJIA) was assessed in WA28117 (NCT01904279), a 52-week, open-label, multicenter, PK-PD and safety study to determine the appropriate subcutaneous dose of ACTEMRA that achieved comparable PK/PD profiles to the ACTEMRA-IV regimen. PJIA patients aged 1 to 17 years with an inadequate response or inability to tolerate MTX, including patients with well-controlled disease on treatment with ACTEMRA-IV and ACTEMRA-naïve patients with active disease, were treated with subcutaneous ACTEMRA based on body weight.
Patients weighing at or above 30 kg (n = 25) were treated with 162 mg of ACTEMRA-SC every 2 weeks and patients weighing less than 30 kg (n = 27) received 162 mg of ACTEMRA-SC every 3 weeks for 52 weeks. Of these 52 patients, 37 (71%) were naive to ACTEMRA and 15 (29%) had been receiving ACTEMRA-IV and switched to ACTEMRA-SC at baseline.
The efficacy of subcutaneous ACTEMRA in children 2 to 17 years of age is based on pharmacokinetic exposure and extrapolation of the established efficacy of intravenous ACTEMRA in polyarticular JIA patients and subcutaneous ACTEMRA in patients with RA [see Clinical Pharmacology (12.3) and Clinical Studies (14.2 and 14.6)].
14.8 Systemic Juvenile Idiopathic Arthritis – Intravenous Administration
The efficacy of ACTEMRA for the treatment of active SJIA was assessed in WA18221 (NCT00642460), a 12-week randomized, double blind, placebo-controlled, parallel group, 2-arm study. Patients treated with or without MTX, were randomized (ACTEMRA:placebo = 2:1) to one of two treatment groups: 75 patients received ACTEMRA infusions every two weeks at either 8 mg per kg for patients at or above 30 kg or 12 mg per kg for patients less than 30 kg and 37 were randomized to receive placebo infusions every two weeks. Corticosteroid tapering could occur from week six for patients who achieved a JIA ACR 70 response. After 12 weeks or at the time of escape, due to disease worsening, patients were treated with ACTEMRA in the open-label extension phase at weight appropriate dosing.
The primary endpoint was the proportion of patients with at least 30% improvement in JIA ACR core set (JIA ACR 30 response) at Week 12 and absence of fever (no temperature at or above 37.5°C in the preceding 7 days). JIA ACR (American College of Rheumatology) responses are defined as the percentage improvement (e.g., 30%, 50%, 70%) in 3 of any 6 core outcome variables compared to baseline, with worsening in no more than 1 of the remaining variables by 30% or more. Core outcome variables consist of physician global assessment, parent per patient global assessment, number of joints with active arthritis, number of joints with limitation of movement, erythrocyte sedimentation rate (ESR), and functional ability (childhood health assessment questionnaire-CHAQ).
Primary endpoint result and JIA ACR response rates at Week 12 are shown in Table 11.
| ACTEMRA N=75 | Placebo N=37 |
|
|---|---|---|
|
||
| Primary Endpoint: JIA ACR 30 response + absence of fever | ||
| Responders | 85% | 24% |
| Weighted difference (95% CI) | 62 (45, 78) | - |
| JIA ACR Response Rates at Week 12 | ||
| JIA ACR 30 | ||
| Responders | 91% | 24% |
| Weighted difference*
(95% CI)† | 67 (51, 83) | - |
| JIA ACR 50 | ||
| Responders | 85% | 11% |
| Weighted difference*
(95% CI) † | 74 (58, 90) | - |
| JIA ACR 70 | ||
| Responders | 71% | 8% |
| Weighted difference*
(95% CI) † | 63 (46, 80) | - |
The treatment effect of ACTEMRA was consistent across all components of the JIA ACR response core variables. JIA ACR scores and absence of fever responses in the open label extension were consistent with the controlled portion of the study (data available through 44 weeks).
14.9 Systemic Juvenile Idiopathic Arthritis – Subcutaneous Administration
Subcutaneously administered ACTEMRA in pediatric patients with systemic juvenile idiopathic arthritis (SJIA) was assessed in WA28118 (NCT01904292), a 52-week, open-label, multicenter, PK-PD and safety study to determine the appropriate subcutaneous dose of ACTEMRA that achieved comparable PK/PD profiles to the ACTEMRA-IV regimen.
Eligible patients received ACTEMRA subcutaneously dosed according to body weight, with patients weighing at or above 30 kg (n = 26) dosed with 162 mg of ACTEMRA every week and patients weighing below 30 kg (n = 25) dosed with 162 mg of ACTEMRA every 10 days (n=8) or every 2 weeks (n=17) for 52 weeks. Of these 51 patients, 26 (51%) were naive to subcutaneous ACTEMRA and 25 (49%) had been receiving ACTEMRA intravenously and switched to subcutaneous ACTEMRA at baseline.
The efficacy of subcutaneous ACTEMRA in children 2 to 17 years of age is based on pharmacokinetic exposure and extrapolation of the established efficacy of intravenous ACTEMRA in systemic JIA patients [see Clinical Pharmacology (12.3) and Clinical Studies (14.8)].
14.10 Cytokine Release Syndrome – Intravenous Administration
The efficacy of ACTEMRA for the treatment of CRS was assessed in a retrospective analysis of pooled outcome data from clinical trials of CAR T-cell therapies for hematological malignancies. Evaluable patients had been treated with tocilizumab 8 mg/kg (12 mg/kg for patients < 30 kg) with or without additional high-dose corticosteroids for severe or life-threatening CRS; only the first episode of CRS was included in the analysis. The study population included 24 males and 21 females (total 45 patients) of median age 12 years (range, 3–23 years); 82% were Caucasian. The median time from start of CRS to first dose of tocilizumab was 4 days (range, 0-18 days). Resolution of CRS was defined as lack of fever and off vasopressors for at least 24 hours. Patients were considered responders if CRS resolved within 14 days of the first dose of tocilizumab, if no more than 2 doses of tocilizumab were needed, and if no drugs other than tocilizumab and corticosteroids were used for treatment. Thirty-one patients (69%; 95% CI: 53%–82%) achieved a response. Achievement of resolution of CRS within 14 days was confirmed in a second study using an independent cohort that included 15 patients (range: 9–75 years old) with CAR T cell-induced CRS.
14.11 COVID-19 – Intravenous Administration
The efficacy of ACTEMRA for the treatment of COVID-19 was based on RECOVERY (NCT04381936), a randomized, controlled, open-label, platform study, and supported by the results from EMPACTA (NCT04372186), a randomized, double-blind, placebo-controlled study. Results of two other randomized, double-blind, placebo-controlled studies, COVACTA (NCT04320615) and REMDACTA (NCT04409262), which evaluated the efficacy of ACTEMRA for the treatment of COVID-19 are also summarized.
RECOVERY (Randomised Evaluation of COVID-19 Therapy) Collaborative Group Study in Hospitalized Adults Diagnosed with COVID-19
RECOVERY was a randomized, controlled, open-label, multicenter platform study conducted in the United Kingdom to evaluate the efficacy and safety of potential treatments in hospitalized adult patients with severe COVID-19 pneumonia. Eligible patients for the ACTEMRA portion of the study had clinically suspected or laboratory-confirmed SARS-CoV-2 infection and no medical contraindications to any of the treatments and had clinical evidence of progressive COVID-19 (defined as oxygen saturation <92% on room air or receiving oxygen therapy, and CRP ≥75 mg/L). Patients were then randomized to receive either standard of care (SoC) or intravenous ACTEMRA at a weight-tiered dosing comparable to the recommended dosage [see Clinical Pharmacology (12.3)] in addition to SoC.
Efficacy analyses were performed in the intent-to-treat (ITT) population comprising 4116 adult patients who were randomized to the ACTEMRA + SoC arm (n=2022) or to the SoC arm (n=2094). The mean age of participants was 64 years (range: 20 to 101), and patients were 67% male, 76% White, 11% Asian, 3% Black or African American, and 1% mixed race. At baseline, 0.2% of patients were not on supplemental oxygen, 45% of patients required low flow oxygen, 41% of patients required non-invasive ventilation or high-flow oxygen, and 14% of patients required invasive mechanical ventilation; 82% of patients were reported to be receiving systemic corticosteroids.
The primary efficacy endpoint was time to death through Day 28. The results for the overall population and the subgroups of patients who were or were not receiving systemic corticosteroids at time of randomization are summarized in Table 12.
| ACTEMRA+ SoC N=2022 n (%)* | SoC N=2094 n (%)* | Hazard Ratio (95% CI) | Risk Difference (95% CI) |
|
|---|---|---|---|---|
|
||||
| Mortality | 621 (30.7%) | 729 (34.9%) | 0.85 (0.76, 0.94) p= 0.0028* | -4.1% (-7.0, -1.3) |
| By baseline receipt of corticosteroid use | ||||
| Mortality for patients receiving systemic corticosteroids at randomization† | 482/1664 (29.0%) | 600/1721 (34.9%) | 0.79 (0.70, 0.89) | -5.9% (-9.1, -2.8) |
| Mortality for patients not receiving systemic corticosteroids at randomization† | 139/357 (39.0%) | 127/367 (34.6%) | 1.16 (0.91, 1.48) | 4.4% (-2.6, 11.5) |
REMDACTA
REMDACTA was a randomized, double-blind, placebo-controlled, multicenter study to evaluate intravenous ACTEMRA 8 mg/kg in combination with intravenous remdesivir (RDV) 200 mg on Day 1 followed by 100 mg once daily for a total of 10 days in hospitalized patients with severe COVID-19 pneumonia. The study randomized 649 adult patients with SARS-CoV-2 infection confirmed by a positive polymerase chain reaction (PCR) result, pneumonia confirmed by radiography, and who required supplemental oxygen > 6 L/min to maintain SpO2 >93%. At baseline, 7% of patients were on low flow oxygen, 80% were on non-invasive ventilation or high flow oxygen, 14% were on invasive mechanical ventilation, and 84% were on corticosteroids.
The primary efficacy endpoint was time from randomization to hospital discharge or 'ready for discharge' up to Day 28. There was no statistically significant difference between the treatment arms with respect to time to hospital discharge or "ready for discharge" through Day 28.
Mortality at Day 28 was 18.1% in the ACTEMRA+ RDV arm versus 19.5% in the placebo +RDV arm (weighted difference (ACTEMRA arm - placebo arm): -1.3% [95% CI, -7.8% to 5.2%]).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
| Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: 12/2022 | ||
|
Medication Guide
|
|||
|
What is the most important information I should know about ACTEMRA? ACTEMRA can cause serious side effects including:
If you have COVID-19, your healthcare provider should monitor you for signs and symptoms of new infections during and after treatment with ACTEMRA. Your healthcare provider should monitor you closely for signs and symptoms of TB during treatment with ACTEMRA.
Before starting ACTEMRA, tell your healthcare provider if you:
|
|||
|
|
|
|
After starting ACTEMRA, call your healthcare provider right away if you have any symptoms of an infection. ACTEMRA can make you more likely to get infections or make worse any infection that you have. If you have COVID-19, your healthcare provider should monitor you for signs and symptoms of new infections during and after treatment with ACTEMRA.
|
|||
|
|
||
See "What are the possible side effects with ACTEMRA?" for more information about side effects. |
|||
|
What is ACTEMRA? ACTEMRA is a prescription medicine called an Interleukin-6 (IL-6) receptor antagonist. ACTEMRA is used:
It is not known if ACTEMRA is safe and effective in children with PJIA, SJIA, or CRS under 2 years of age or in children with conditions other than PJIA, SJIA or CRS. |
|||
|
Do not take ACTEMRA: if you are allergic to tocilizumab, or any of the ingredients in ACTEMRA. See the end of this Medication Guide for a complete list of ingredients in ACTEMRA. |
|||
|
Before you receive ACTEMRA, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all of the medicines you take, including prescription, over-the-counter medicines, vitamins and herbal supplements. ACTEMRA and other medicines may affect each other causing side effects. Especially tell your healthcare provider if you take:
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. |
|||
|
How will I receive ACTEMRA? Into a vein (IV or intravenous infusion) for Rheumatoid Arthritis, Giant Cell Arteritis, PJIA, SJIA, CRS or COVID-19:
Under the skin (SC or subcutaneous injection) for Rheumatoid Arthritis, Giant Cell Arteritis, SSc-ILD, PJIA or SJIA:
|
|||
|
What are the possible side effects with ACTEMRA? ACTEMRA can cause serious side effects, including:
|
|||
|
|
|
|
The most common side effects of ACTEMRA include:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Genentech at 1-888-835-2555. |
|||
|
General information about the safe and effective use of ACTEMRA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not give ACTEMRA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ACTEMRA that is written for health professionals. |
|||
|
What are the ingredients in ACTEMRA? Active ingredient: tocilizumab. Inactive ingredients of Intravenous ACTEMRA: disodium phosphate dodecahydrate/sodium dihydrogen phosphate dihydrate buffered solution, polysorbate 80, sucrose, and water for Injection. Inactive ingredients of Subcutaneous ACTEMRA: L-arginine hydrochloride, L-histidine, L-histidine hydrochloride monohydrate, L-methionine, polysorbate 80, and water for Injection. ACTEMRA is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group. ACTPen is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
Genentech, Inc.,
|
|||
Instructions for Use
ACTEMRA® (AC-TEM-RA)
(tocilizumab)
Injection, For Subcutaneous Use
Single-dose Prefilled Syringe
Read and follow the Instructions for Use that come with your ACTEMRA prefilled syringe before you start using it and each time you get a prescription refill. Before you use ACTEMRA prefilled syringe for the first time, make sure your healthcare provider shows you the right way to use it.
- Do not remove the needle cap until you are ready to inject ACTEMRA.
- Do not try to take apart the syringe at any time.
- Do not reuse the same syringe.
Parts of your ACTEMRA Prefilled Syringe (See Figure A).
Supplies needed for your ACTEMRA Prefilled Syringe Injection (See Figure B):
- ACTEMRA prefilled syringe
- alcohol pad
- sterile cotton ball or gauze
- puncture-resistant container or sharps container for safe disposal of needle cap and used syringe (See Step 4 "Dispose of the syringe")
Step 1. Preparing for an ACTEMRA Injection
Find a comfortable space with a clean, flat, working surface.
- Take the box containing the syringe out of the refrigerator and open the box. Do not touch the trigger fingers on the syringe as this may damage the syringe.
- Remove 1 single-use ACTEMRA prefilled syringe from the box and let it warm up for 30 minutes to allow it to reach room temperature. If the syringe does not reach room temperature, this could cause your injection to feel uncomfortable and make it difficult to push the plunger in.
- Do not speed up the warming process in any way, such as using the microwave or placing the syringe in warm water.
- Check the expiration date on the ACTEMRA prefilled syringe (See Figure A). Do not use it if the expiration date has passed because it may not be safe to use. If the expiration date has passed safely dispose of the syringe in a sharps container and get a new one.
Do not remove the needle cap while allowing your ACTEMRA prefilled syringe to reach room temperature.
- Keep your unused syringes in the original carton and keep in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
- Once removed from the refrigerator, your prefilled syringe can be stored up to 2 weeks at or below 86°F (30°C). Your prefilled syringe must always be kept in the original carton in order to protect from light and moisture. Hold your ACTEMRA prefilled syringe with the covered needle pointing down (See Figure C).
- Check the liquid in the ACTEMRA prefilled syringe. It should be clear and colorless to pale yellow. Do not inject ACTEMRA if the liquid is cloudy, discolored, or has lumps or particles in it because it may not be safe to use. Safely dispose of the syringe in a sharps container and get a new one.
- Wash your hands well with soap and water.
Step 2. Choose and Prepare an Injection Site
Choose an Injection Site
- The front of your thigh and your abdomen except for the 2-inch area around your navel are the recommended injection sites (See Figure D).
- The outer area of the upper arms may also be used only if the injection is being given by a caregiver. Do not attempt to use the upper arm area by yourself (See Figure D).
Rotate Injection Site
- Choose a different injection site for each new injection at least 1-inch from the last area you injected.
- Do not inject into moles, scars, bruises, or areas where the skin is tender, red, hard or not intact.
Prepare the Injection Site
- Wipe the injection site with an alcohol pad in a circular motion and let it air dry to reduce the chance of getting an infection. Do not touch the injection site again before giving the injection.
- Do not fan or blow on the clean area.
Step 3. Inject ACTEMRA
- Hold the ACTEMRA prefilled syringe with 1 hand and pull the needle cap straight off with your other hand (See Figure E). Do not hold the plunger while you remove the needle cap. If you cannot remove the needle cap you should ask a caregiver for help or contact your healthcare provider.
- Throw away the needle cap in a sharps container.
- There may be a small air bubble in the ACTEMRA prefilled syringe. You do not need to remove it.
- You may see a drop of liquid at the end of the needle. This is normal and will not affect your dose.
- Do not touch the needle or let it touch any surfaces.
- Do not use the prefilled syringe if it is dropped.
- If it is not used within 5 minutes of needle cap removal, the syringe should be disposed of in the puncture resistant container or sharps container and a new syringe should be used.
- Never reattach the needle cap after removal.
- Hold the ACTEMRA prefilled syringe in 1 hand between the thumb and index finger (See Figure F).
- Do not pull back on the plunger of the syringe.
- Use your other hand and gently pinch the area of skin you cleaned. Hold the pinched skin firmly. Pinching the skin is important to make sure that you inject under the skin (into fatty tissue) but not any deeper (into muscle). Injection into muscle could cause the injection to feel uncomfortable.
- Do not hold or push on the plunger while inserting the needle into the skin.
- Use a quick, dart-like motion to insert the needle all the way into the pinched skin at an angle between 45° to 90° (See Figure G). It is important to use the correct angle to make sure the medicine is delivered under the skin (into fatty tissue), or the injection could be painful and the medicine may not work.
- Keep the syringe in position and let go of the pinch of skin.
- Slowly inject all of the medicine by gently pushing the plunger all the way down (See Figure H). You must press the plunger all the way down to get the full dose of medicine and to ensure the trigger fingers are completely pushed to the side. If the plunger is not fully depressed the needle shield will not extend to cover the needle when it is removed. If the needle is not covered, carefully place the syringe into the puncture resistant container to avoid injury with the needle.
- After the plunger is pushed all the way down, keep pressing down on the plunger to be sure all of the medicine is injected before taking the needle out of the skin.
- Keep pressing down on the plunger while you take the needle out of the skin at the same angle as inserted (See Figure I).
- After the needle is removed completely from the skin, release the plunger, allowing the needle-shield to protect the needle (See Figure J).
After the Injection
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site.
- Do not rub the injection site.
- If needed, you may cover the injection site with a small bandage.
Step 4. Dispose of the syringe
- The ACTEMRA prefilled syringe should not be reused.
- Put the used syringe into your puncture resistant container (See "How do I throw away used syringes?")
- Do not put the needle cap back on the needle.
- If your injection is given by another person, this person must also be careful when removing the syringe and disposing of the syringe to prevent accidental needle stick injury and passing infection.
How do I throw away used syringes?
- Put your used needles and syringes including ACTEMRA in a FDA-cleared sharps disposal container right away after use (See Figure K). Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Keep ACTEMRA prefilled syringes and the disposal container out of the reach of children.
Record your Injection
- Write the date, time, and specific part of your body where you injected yourself. It may also be helpful to write any questions or concerns about the injection so you can ask your healthcare provider.
If you have questions or concerns about your ACTEMRA prefilled syringe, please contact your healthcare provider familiar with ACTEMRA or call 1-800-ACTEMRA.
This Medication Guide and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Medication Guide Revised: 06/2022
ACTEMRA is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
U.S. License No.: 1048
© 2022 Genentech, Inc. All rights reserved.
Instructions for Use
ACTEMRA® (AC-TEM-RA)
(tocilizumab)
Injection, For Subcutaneous Use
Single-dose Prefilled ACTPen® (AKT-PEN) Autoinjector
Read and follow the Instructions for Use that come with your ACTEMRA ACTPen autoinjector before you start using it and each time you get a prescription refill. Before you use the ACTEMRA ACTPen autoinjector for the first time, make sure your healthcare provider shows you the right way to use it.
Important: Keep your unused Autoinjectors in the original carton and keep in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze. Once removed from the refrigerator, your Autoinjector can be stored up to 2 weeks at or below 86°F (30°C). Your Autoinjector must always be kept in the original carton in order to protect from light and moisture.
- Do not remove the Autoinjector cap until you are ready to inject ACTEMRA.
- Do not try to take apart the Autoinjector at any time.
- Do not reuse the same Autoinjector.
- Do not use the Autoinjector through clothing.
- Do not leave the Autoinjector unattended.
- Do not use the Autoinjector if it appears to be damaged or if you have accidentally dropped the Autoinjector.
- Keep out of the reach of children.
Parts of your ACTEMRA ACTPen autoinjector (See Figure A).
Supplies needed for an injection using your ACTEMRA ACTPen autoinjector (See Figure B):
- 1 ACTEMRA ACTPen autoinjector
- 1 Alcohol pad
- 1 Sterile cotton ball or gauze
- 1 Puncture-resistant container or sharps container for safe disposal of Autoinjector cap and used Autoinjector (See Step 4 "Dispose of the Autoinjector")
Step 1. Preparing for an ACTEMRA Injection
Find a comfortable space with a clean, flat, working surface.
- Take the box containing the Autoinjector out of the refrigerator.
- If you are opening the box for the first time, check to make sure that it is properly sealed. Do not use the Autoinjector if the box looks like it has already been opened.
- Check that the Autoinjector box is not damaged. Do not use ACTEMRA ACTPen autoinjector if the box looks damaged.
- Check the expiration date on the Autoinjector box. Do not use the Autoinjector if the expiration date has passed because it may not be safe to use.
- Open the box, and remove 1 single-use ACTEMRA ACTPen autoinjector from the box.
- Return any remaining autoinjectors in the box to the refrigerator.
- Check the expiration date on the ACTEMRA ACTPen autoinjector (See Figure A). Do not use it if the expiration date has passed because it may not be safe to use. If the expiration date has passed, safely dispose of the Autoinjector in a sharps container and get a new one.
- Check the Autoinjector to make sure it is not damaged. Do not use the Autoinjector if it appears to be damaged or if you have accidentally dropped the Autoinjector.
- Place the Autoinjector on a clean, flat surface and let the Autoinjector warm up for 45 minutes to allow it to reach room temperature. If the Autoinjector does not reach room temperature, this could cause your injection to feel uncomfortable and it could take longer to inject.
- -
- Do not speed up the warming process in any way, such as using the microwave or placing the Autoinjector in warm water.
- -
- Do not leave the Autoinjector to warm up in direct sunlight.
- -
- Do not remove the green cap while allowing your ACTEMRA ACTPen autoinjector to reach room temperature.
- Hold your ACTEMRA ACTPen autoinjector with the green cap pointing down (See Figure C).
- Look in the clear Window area. Check the liquid in the ACTEMRA ACTPen autoinjector (See Figure C). It should be clear and colorless to pale yellow. Do not inject ACTEMRA if the liquid is cloudy, discolored, or has lumps or particles in it because it may not be safe to use. Safely dispose of the Autoinjector in a sharps container and get a new one.
- Wash your hands well with soap and water.
Step 2. Choose and Prepare an Injection Site
Choose an Injection Site
- The front of your thigh or your abdomen except for the 2-inch (5cm) area around your navel are the recommended injection sites (See Figure D).
- The outer area of the upper arms may also be used only if the injection is being given by a caregiver. Do not attempt to use the upper arm area by yourself (See Figure D).
Rotate Injection Site
- Choose a different injection site for each new injection at least 1 inch (2.5cm) from the last area you injected.
- Do not inject into moles, scars, bruises, or areas where the skin is tender, red, hard or not intact.
Prepare the Injection Site
- Wipe the injection site with an alcohol pad in a circular motion and let it air dry to reduce the chance of getting an infection. Do not touch the injection site again before giving the injection.
- Do not fan or blow on the clean area.
Step 3. Inject ACTEMRA
- Hold the ACTEMRA ACTPen autoinjector firmly with one hand. Twist and pull off the green cap with the other hand (See Figure E). The green cap contains a loose fitting metal tube.
- If you cannot remove the green cap you should ask a caregiver for help or contact your healthcare provider.
Important: Do not touch the needle shield which is located at the tip of the Autoinjector below the Window area (See Figure A), to avoid accidental needle stick injury. - Throw away the green cap in a sharps container.
- After you remove the green cap, the Autoinjector is ready for use. If the Autoinjector is not used within 3 minutes of the cap removal, the Autoinjector should be disposed of in the sharps container and a new Autoinjector should be used.
- Never reattach the green cap after removal.
- Hold the Autoinjector comfortably in 1 hand by the upper part, so that you can see the Window area of the Autoinjector (See Figure F).
- Use your other hand to gently pinch the area of skin you cleaned, to prepare a firm injection site (See Figure G). The Autoinjector requires a firm injection site to properly activate. Pinching the skin is important to make sure that you inject under the skin (into fatty tissue) but not any deeper (into muscle). Injection into muscle could cause the injection to feel uncomfortable.
-
Do not press the green Activation button yet.
Place the needle-shield of the Autoinjector against your pinched skin at a 90° angle (See Figure H). - It is important to use the correct angle to make sure the medicine is delivered under the skin (into fatty tissue), or the injection could be painful and the medicine may not work.
- Continue to gently pinch throughout the injection procedure.
- To use the Autoinjector, you first have to unlock the green Activation button. To unlock it, press the Autoinjector firmly against your pinched skin until the needle-shield is completely pushed in (See Figure I).
- Continue to keep the needle-shield pushed in. If you do not keep the needle-shield completely pushed against the skin, the green Activation button will not work. Continue to pinch the skin while you keep the Autoinjector in place.
- Press the green Activation button to start the injection. A "click" sound indicates the start of the injection. Keep the green button pressed in and continue holding the Autoinjector pressed firmly against your skin (See Figure J). If you cannot start the injection you should ask for help from a caregiver or contact your healthcare provider.
- The purple indicator will move along the Window area during the injection (See Figure K).
- Watch the purple indicator until it stops moving to be sure the full dose of medicine is injected. This may take up to 10 seconds.
- You may hear a second "click" during the injection but you should continue to hold the Autoinjector firmly against your skin until the purple indicator stops moving.
- When the purple indicator has stopped moving, release the green button. Lift the Autoinjector straight off of the injection site at a 90° angle to remove the needle from the skin. The needle-shield will then move out and lock into place covering the needle (See Figure L).
- Check the Window area to see that it is filled with the purple indicator (See Figure L).
- If the Window area is not filled by the purple indicator then:
- The needle-shield may not have locked. Do not touch the needle-shield of the Autoinjector, because you may stick yourself with the needle. If the needle is not covered, carefully place the Autoinjector into the sharps container to avoid any injury with the needle.
- You may not have received your full dose of ACTEMRA. Do not try to re-use the Autoinjector. Do not repeat the injection with another Autoinjector. Call your healthcare provider for help.
After the Injection
- There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site.
- Do not rub the injection site.
- If needed, you may cover the injection site with a small bandage.
Step 4. Dispose of the Autoinjector
- The ACTEMRA ACTPen autoinjector should not be reused.
- Put the used Autoinjector into your sharps container (See "How do I dispose of used Autoinjectors?").
- Do not put the cap back on the Autoinjector.
- If your injection is given by another person, this person must also be careful when removing the Autoinjector and disposing of it to prevent accidental needle stick injury and passing infection.
How do I dispose of used Autoinjectors?
- Put your used ACTEMRA ACTPen autoinjector and green cap in a FDA-cleared sharps disposal container right away after use (See Figure M).
- Do not throw away (dispose of) the Autoinjector and the green cap in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should dispose of used Autoinjectors. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Keep the ACTEMRA ACTPen autoinjector and disposal container out of the reach of children.
Record your Injection
- Write the date, time, and specific part of your body where you injected yourself. It may also be helpful to write any questions or concerns about the injection so you can ask your healthcare provider.
If you have any questions or concerns about your ACTEMRA ACTPen autoinjector, talk to your healthcare provider familiar with ACTEMRA or call 1-800-ACTEMRA.
This Medication Guide and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Medication Guide Revised: 06/2022
ACTEMRA is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
ACTPen is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
U.S. License No.: 1048
© 2022 Genentech, Inc. All rights reserved.
| ACTEMRA
tocilizumab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ACTEMRA
tocilizumab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ACTEMRA
tocilizumab injection, solution, concentrate |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ACTEMRA
tocilizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ACTEMRA ACTPEN
tocilizumab injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Genentech, Inc. (080129000) |
| Registrant - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| F. Hoffmann-La Roche Ltd | 485244961 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) , LABEL(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) , PACK(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 146373191 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) , API MANUFACTURE(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 080129000 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Diagnostics GmbH | 323105205 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Singapore Technical Operations Pte. Ltd. | 937189173 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 833220176 | MANUFACTURE(50242-137) , PACK(50242-137) , LABEL(50242-137) , ANALYSIS(50242-137) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genentech, Inc. | 004074162 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) , API MANUFACTURE(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Roche Diagnostics GmbH | 315028860 | ANALYSIS(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) , PACK(50242-135, 50242-136, 50242-137, 50242-138, 50242-143) | |