Drug Detail:Acular (Ketorolac ophthalmic [ kee-toe-role-ak-off-thal-mik ])
Drug Class: Ophthalmic anti-inflammatory agents
Highlights of Prescribing Information
These highlights do not include all the information needed to use ACULAR® safely and effectively. See full prescribing information for ACULAR®.
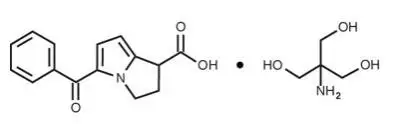
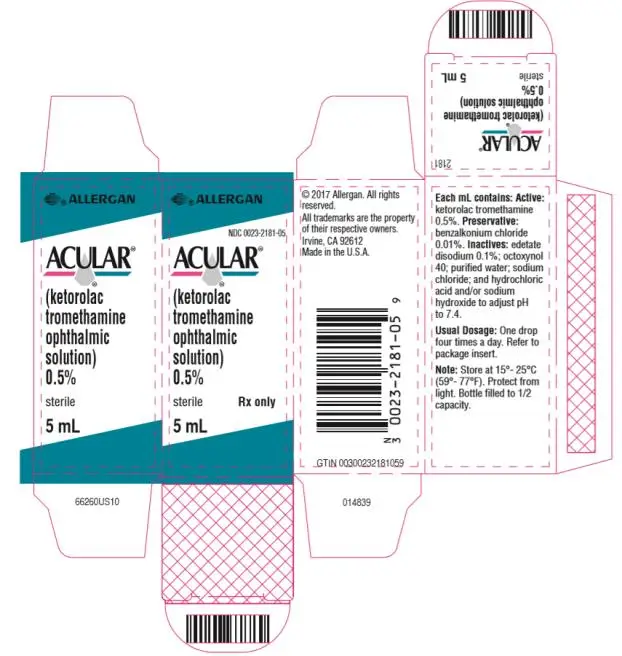
ACULAR® (ketorolac tromethamine ophthalmic solution) 0.5%
Initial U.S. Approval: 1991
Indications and Usage for Acular
ACULAR® ophthalmic solution is a nonsteroidal, anti-inflammatory indicated for:
- The treatment of inflammation following cataract surgery. (1)
- The temporary relief of ocular itching due to seasonal allergic conjunctivitis. (1)
Acular Dosage and Administration
One drop of ACULAR® should be applied to the affected eye(s) four times a day for relief of ocular itching due to seasonal allergic conjunctivitis.
For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ACULAR® should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period. (2.1)
Dosage Forms and Strengths
Ophthalmic solution containing 5 mg/mL ketorolac tromethamine. (3)
- 10 mL size bottle filled with 5 mL of solution
Contraindications
Hypersensitivity to any component of this product. (4)
Warnings and Precautions
- Delayed healing (5.1)
- Cross-sensitivity or hypersensitivity (5.2)
- Increased bleeding time due to interference with thrombocyte aggregation (5.3)
- Corneal effects including keratitis (5.4)
Adverse Reactions/Side Effects
The most frequent adverse reactions reported by up to 40% of patients participating in clinical trials have been transient stinging and burning on instillation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-433-8871 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2012
Related/similar drugs
diclofenac ophthalmic, dexamethasone ophthalmic, ketorolac ophthalmic, prednisolone ophthalmic, Pataday, olopatadine ophthalmic, LotemaxFull Prescribing Information
1. Indications and Usage for Acular
ACULAR® ophthalmic solution is indicated for the temporary relief of ocular itching due to seasonal allergic conjunctivitis. ACULAR® ophthalmic solution is also indicated for the treatment of postoperative inflammation in patients who have undergone cataract extraction.
2. Acular Dosage and Administration
2.1 Recommended Dosing
Patient Dosing
The recommended dose of ACULAR® ophthalmic solution is one drop four times a day to the affected eye(s) for relief of ocular itching due to seasonal allergic conjunctivitis.
For the treatment of postoperative inflammation in patients who have undergone cataract extraction, one drop of ACULAR® ophthalmic solution should be applied to the affected eye four times daily beginning 24 hours after cataract surgery and continuing through the first 2 weeks of the postoperative period.
3. Dosage Forms and Strengths
10 mL size bottle filled with 5 mL of ketorolac tromethamine ophthalmic solution, 0.5% (5 mg/mL)
4. Contraindications
ACULAR® solution is contraindicated in patients with previously demonstrated hypersensitivity to any of the ingredients in the formulation.
5. Warnings and Precautions
5.1 Delayed Healing
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.2 Cross-Sensitivity or Hypersensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs. There have been reports of bronchospasm or exacerbation of asthma associated with the use of ketorolac tromethamine ophthalmic solution in patients who have either a known hypersensitivity to aspirin/non-steroidal anti-inflammatory drugs or a past medical history of asthma. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5.3 Increased Bleeding Time
With some NSAIDs, there exists the potential for increased bleeding time due to interference with thrombocyte aggregation. There have been reports that ocularly applied nonsteroidal anti-inflammatory drugs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that ACULAR® ophthalmic solution be used with caution in patients with known bleeding tendencies or who are receiving other medications, which may prolong bleeding time.
5.4 Corneal Effects
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration, or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 1 day prior to surgery or use beyond 14 days post-surgery may increase patient risk for the occurrence and severity of corneal adverse events.
6. Adverse Reactions/Side Effects
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.1 Clinical Studies Experience
The most frequent adverse reactions reported with the use of ketorolac tromethamine ophthalmic solutions have been transient stinging and burning on instillation. These reactions were reported by up to 40% of patients participating in clinical trials.
Other adverse reactions occurring approximately 1 to 10% of the time during treatment with ketorolac tromethamine ophthalmic solutions included allergic reactions, corneal edema, iritis, ocular inflammation, ocular irritation, superficial keratitis, and superficial ocular infections.
Other adverse reactions reported rarely with the use of ketorolac tromethamine ophthalmic solutions included: corneal infiltrates, corneal ulcer, eye dryness, headaches, and visual disturbance (blurry vision).
| ACULAR
ketorolac tromethamine solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |