Drug Detail:Aczone (Dapsone topical [ dap-sone-top-i-kal ])
Drug Class: Topical acne agents
Highlights of Prescribing Information
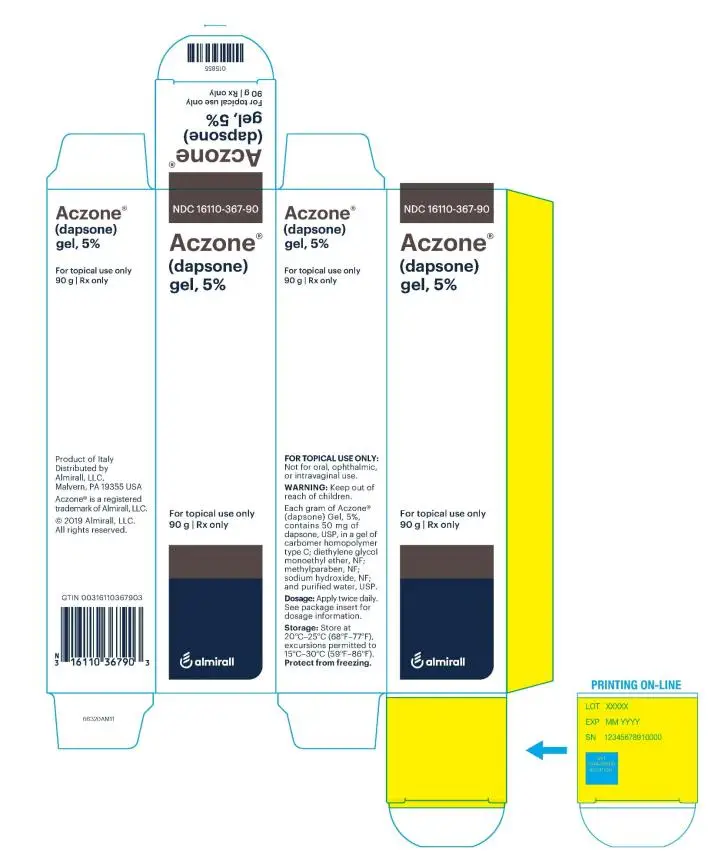
ACZONE® (dapsone) Gel, 5%, for topical use
Initial U.S. Approval: 1955
Indications and Usage for Aczone
ACZONE® Gel is indicated for the topical treatment of acne vulgaris (1).
Aczone Dosage and Administration
- Apply twice daily (2).
- Apply approximately a pea-sized amount of ACZONE Gel, 5%, in a thin layer to the acne affected area (2).
- If there is no improvement after 12 weeks, treatment with ACZONE Gel, 5%, should be reassessed (2).
- For topical use only. Not for oral, ophthalmic, or intravaginal use (2).
Dosage Forms and Strengths
Gel, 5% (3).
Contraindications
None (4).
Warnings and Precautions
- Methemoglobinemia: Cases of methemoglobinemia have been reported. Discontinue ACZONE gel if signs of methemoglobinemia occur (5.1).
- Hematologic Effects: Some subjects with G6PD deficiency using ACZONE Gel developed laboratory changes suggestive of hemolysis. (5.2)(8.6).
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 10%) are oiliness/peeling, dryness and erythema at the application site (6).
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Trimethoprim/sulfamethoxazole (TMP/SMX) increases the level of dapsone and its metabolites (7.1).
- Topical benzoyl peroxide used at the same time as ACZONE may result in temporary local yellow or orange skin discoloration (7.2).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2019
Full Prescribing Information
1. Indications and Usage for Aczone
ACZONE® Gel, 5%, is indicated for the topical treatment of acne vulgaris.
2. Aczone Dosage and Administration
For topical use only. Not for oral, ophthalmic, or intravaginal use.
After the skin is gently washed and patted dry, apply approximately a pea-sized amount of ACZONE Gel, 5%, in a thin layer to the acne affected areas twice daily. Rub in ACZONE Gel, 5%, gently and completely. ACZONE Gel, 5%, is gritty with visible drug substance particles. Wash hands after application of ACZONE Gel, 5%.
If there is no improvement after 12 weeks, treatment with ACZONE Gel, 5%, should be reassessed.
3. Dosage Forms and Strengths
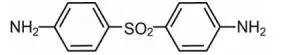
Gel, 5%. Each gram of ACZONE gel contains 50 mg of dapsone in a white to pale yellow gel.
5. Warnings and Precautions
5.1 Methemoglobinemia
Cases of methemoglobinemia, with resultant hospitalization, have been reported postmarketing in association with ACZONE Gel, 5% treatment. Patients with glucose‐6‐phosphate dehydrogenase deficiency or congenital or idiopathic methemoglobinemia are more susceptible to drug‐induced methemoglobinemia. Avoid use of ACZONE Gel, 5% in those patients with congenital or idiopathic methemoglobinemia.
Signs and symptoms of methemoglobinemia may be delayed some hours after exposure. Initial signs and symptoms of methemoglobinemia are characterized by a slate grey cyanosis seen in, e.g., buccal mucous membranes, lips and nail beds. Advise patients to discontinue ACZONE Gel, 5% and seek immediate medical attention in the event of cyanosis.
Dapsone can cause elevated methemoglobin levels particularly in conjunction with methemoglobin‐inducing agents.
5.2 Hematologic Effects
Oral dapsone treatment has produced dose-related hemolysis and hemolytic anemia. Individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency are more prone to hemolysis with the use of certain drugs. G6PD deficiency is most prevalent in populations of African, South Asian, Middle Eastern, and Mediterranean ancestry.
Some subjects with G6PD deficiency using ACZONE Gel developed laboratory changes suggestive of hemolysis. There was no evidence of clinically relevant hemolysis or anemia in patients treated with ACZONE Gel, 5%, including patients who were G6PD deficient.
Discontinue ACZONE Gel, 5%, if signs and symptoms suggestive of hemolytic anemia occur. Avoid use of ACZONE Gel, 5% in patients who are taking oral dapsone or antimalarial medications because of the potential for hemolytic reactions. Combination of ACZONE Gel, 5%, with trimethoprim/sulfamethoxazole (TMP/SMX) may increase the likelihood of hemolysis in patients with G6PD deficiency.
7. Drug Interactions
7.1 Trimethoprim-Sulfamethoxazole
A drug-drug interaction study evaluated the effect of the use of ACZONE Gel, 5%, in combination with double strength (160 mg/800 mg) trimethoprim-sulfamethoxazole (TMP/SMX). During co-administration, systemic levels of TMP and SMX were essentially unchanged. However, levels of dapsone and its metabolites increased in the presence of TMP/SMX. Systemic exposure (AUC0-12) of dapsone and N-acetyl-dapsone (NAD) were increased by about 40% and 20% respectively in the presence of TMP/SMX. Notably, systemic exposure (AUC0-12) of dapsone hydroxylamine (DHA) was more than doubled in the presence of TMP/SMX. Exposure from the proposed topical dose is about 1% of that from the 100 mg oral dose, even when co-administered with TMP/SMX.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no available data on ACZONE Gel, 5%, use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. In animal reproduction studies, oral doses of dapsone administered to pregnant rats and rabbits during organogenesis that resulted in systemic exposures more than 250 times the systemic exposure at the maximum recommended human dose (MRHD) of ACZONE Gel, 5%, resulted in embryocidal effects. When orally administered to rats from the onset of organogenesis through the end of lactation at systemic exposures approximately 400 times the exposure at the MRHD, dapsone resulted in increased stillbirths and decreased pup weight [see Data].
The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Dapsone has been shown to have an embryocidal effect in rats and rabbits when administered orally daily to females during organogenesis at dosages of 75 mg/kg/day and 150 mg/kg/day, respectively. These dosages resulted in systemic exposures that represented approximately 956 times [rats] and 289 times [rabbits] the systemic exposure observed in human females as a result of use of the MRHD of ACZONE Gel, 5%, based on AUC comparisons. These effects were probably secondary to maternal toxicity.
Dapsone was assessed for effects on perinatal/postnatal pup development and postnatal maternal behavior and function in a study in which dapsone was orally administered to female rats daily beginning on the seventh day of gestation and continuing until the twenty-seventh day postpartum. Maternal toxicity (decreased body weight and food consumption) and developmental effects (increase in stillborn pups and decreased pup weight) were seen at a dapsone dose of 30 mg/kg/day (approximately 382 times the systemic exposure that is associated with the MRHD of ACZONE Gel, 5%, based on AUC comparisons). No effects were observed on the viability, physical development, behavior, learning ability, or reproductive function of surviving pups.
8.6 G6PD Deficiency
ACZONE Gel, 5% and vehicle were evaluated in a randomized, double-blind, cross-over design clinical trial of 64 subjects with G6PD deficiency and acne vulgaris. Subjects were Black (88%), Asian (6%), Hispanic (2%) or of other racial origin (5%). Blood samples were taken at Baseline, Week 2, and Week 12 during both vehicle and ACZONE Gel, 5% treatment periods. There were 56 out of 64 subjects who had a Week 2 blood draw and applied at least 50% of treatment applications. Table 3 contains results from testing of relevant hematology parameters for these two treatment periods. ACZONE Gel was associated with a 0.32 g/dL drop in hemoglobin after two weeks of treatment, but hemoglobin levels generally returned to baseline levels at Week 12.
| ACZONE® | Vehicle | ||||
| N | Mean | N | Mean | ||
| Hemoglobin (g/dL) | Pre-treatment | 53 | 13.44 | 56 | 13.36 |
| 2 weeks | 53 | 13.12 | 55 | 13.34 | |
| 12 weeks | 50 | 13.42 | 50 | 13.37 | |
| Bilirubin (mg/dL) | Pre-treatment | 54 | 0.58 | 56 | 0.55 |
| 2 weeks | 53 | 0.65 | 55 | 0.56 | |
| 12 weeks | 50 | 0.61 | 50 | 0.62 | |
| Reticulocytes (%) | Pre-treatment | 53 | 1.30 | 55 | 1.34 |
| 2 weeks | 53 | 1.51 | 55 | 1.34 | |
| 12 weeks | 50 | 1.48 | 50 | 1.41 | |
There were no changes from baseline in haptoglobin or lactate dehydrogenase during ACZONE or vehicle treatment at either the 2-week or 12-week time point.
The proportion of subjects who experienced decreases in hemoglobin ≥1 g/dL was similar between ACZONE Gel, 5% and vehicle treatment (8 of 58 subjects had such decreases during ACZONE treatment compared to 7 of 56 subjects during vehicle treatment among subjects with at least one on-treatment hemoglobin assessment). Subgroups based on gender, race, or G6PD enzyme activity did not display any differences in laboratory results from the overall study group. There was no evidence of clinically significant hemolytic anemia in this study. Some of these subjects developed laboratory changes suggestive of hemolysis.
| PATIENT INFORMATION
ACZONE® (AK-zōn) (dapsone) Gel, 5% |
||
| Important: For use on skin only (topical use). Do not use ACZONE® Gel, 5% in or on your mouth, eyes, or vagina. | ||
| What is ACZONE® Gel, 5%?
ACZONE® Gel, 5% is a prescription medicine used on your skin (topical) to treat acne vulgaris. ACZONE® Gel has not been studied in children under 12 years of age. |
||
Before using ACZONE® Gel, 5%, tell your doctor about all of your medical conditions, including if you:
|
||
How should I use ACZONE® Gel, 5%?
|
||
| What are the possible side effects of ACZONE® Gel, 5%?
ACZONE® Gel, 5% may cause serious side effects, including:
|
||
|
|
|
| The most common side effects of ACZONE® Gel, 5% include oiliness, peeling, dryness, and redness of the skin being treated. These are not all of the possible side effects of ACZONE® Gel, 5%. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store ACZONE® Gel, 5%?
|
||
| General information about the safe and effective use of ACZONE® Gel, 5%?
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ACZONE® Gel, 5% for a condition for which it was not prescribed. Do not give ACZONE® Gel, 5% to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about ACZONE® Gel, 5% that is written for health professionals. |
||
| What are the ingredients in ACZONE® Gel, 5%?
Active ingredient: dapsone Inactive ingredients: carbomer homopolymer type C, diethylene glycol monoethyl ether, methylparaben, sodium hydroxide, and purified water, USP. Product of Italy Distributed by Alrmirall, LLC, Exton, PA 19341, USAACZONE® is a trademark of Almirall, LLC. Almirall® and its design are trademarks of Almirall, Inc. ©2019 Almirall, LLC. All rights reserved.  v1.0PPI3670 For more information, call 1-800-678-1605 |
||
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 07/2019
| ACZONE
dapsone gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - ALMIRALL, LLC (605425912) |





 ---------------------------------------------------------------------------------------------------------------
---------------------------------------------------------------------------------------------------------------