Drug Detail:Adhansia xr (Methylphenidate (oral) [ meth-il-fen-i-date ])
Drug Class: CNS stimulants
Highlights of Prescribing Information
ADHANSIA XR (methylphenidate hydrochloride) extended-release capsules, for oral use, CII
Initial U.S. Approval: 1955
WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning.
- CNS stimulants, including ADHANSIA XR, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence (5.1, 9.2, 9.3)
- Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy (5.1, 9.2)
Indications and Usage for Adhansia XR
ADHANSIA XR is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older. (1)
Adhansia XR Dosage and Administration
- Recommended starting dose for patients 6 years and older: 25 mg once daily in the morning. (2.2)
- Dosage may be increased in increments of 10 to 15 mg at intervals of at least 5 days. (2.2)
- Dosages above 85 mg daily in adults and 70 mg and above daily in pediatric patients are associated with disproportionate increases in the incidence of certain adverse reactions. (2.2)
- Administer with or without food. (2.2)
- Capsules may be swallowed whole or opened and the entire contents sprinkled onto a tablespoon of applesauce or yogurt. If sprinkled, take the contents without crushing or chewing. (2.2)
- To avoid substitution errors and overdosage, do not substitute for other methylphenidate products on a milligram-per-milligram basis. (2.4)
Dosage Forms and Strengths
Extended-release Capsules: 25 mg, 35 mg, 45 mg, 55 mg, 70 mg and 85 mg (3)
Contraindications
- Known hypersensitivity to methylphenidate or product components. (4)
- Concurrent treatment with a monoamine oxidase inhibitor (MAOI) or use of an MAOI within the preceding 14 days. (4)
Warnings and Precautions
- Serious Cardiovascular Events: Sudden death has been reported in association with CNS stimulants at recommended doses in pediatric patients with structural cardiac abnormalities or other serious heart problems. In adults, sudden death, stroke, and myocardial infarction have been reported. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmias, or coronary artery disease. (5.2)
- Blood Pressure and Heart Rate Increases: Monitor blood pressure and pulse. Consider the benefits and risks in patients for whom an increase in blood pressure or heart rate would be problematic. (5.3)
- Psychiatric Adverse Reactions: Use of CNS stimulants may cause psychotic or manic symptoms in patients with no prior history, or exacerbation of symptoms in patients with pre-existing psychiatric illness. Evaluate for bipolar disorder prior to ADHANSIA XR use. (5.4)
- Priapism: Cases of painful and prolonged penile erections and priapism have been reported with methylphenidate products. Immediate medical attention should be sought if signs or symptoms of prolonged penile erections or priapism are observed. (5.5)
- Peripheral Vasculopathy, including Raynaud’s Phenomenon: Stimulants used to treat ADHD are associated with peripheral vasculopathy, including Raynaud’s phenomenon. Careful observation for digital changes is necessary during treatment with ADHD stimulants. (5.6)
- Long-Term Suppression of Growth: Monitor height and weight at appropriate intervals in pediatric patients. (5.7)
- Allergic-Type Reactions: FD&C Yellow No. 5: ADHANSIA XR 45 mg capsules contain FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. (5.8)
Adverse Reactions/Side Effects
The most common (≥5% and twice the rate of placebo) adverse reactions occurring with ADHANSIA XR in adults are insomnia, dry mouth, nausea, and decreased appetite.
The most common (≥5% and twice the rate of placebo) adverse reactions occurring with ADHANSIA XR in pediatric patients are decreased appetite, insomnia, and weight decreased. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Purdue Pharma L.P. at 1-888-726-7535 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antihypertensive drugs: Monitor blood pressure. Adjust dosage of antihypertensive drug as needed (7.1).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2021
Related/similar drugs
Adderall, Vyvanse, methylphenidate, Strattera, Ritalin, ConcertaFull Prescribing Information
Adhansia XR Dosage and Administration
2.2 General Dosing Information
Administer ADHANSIA XR orally once daily in the morning with or without food.
Dosage Forms and Strengths
-
25 mg (methylphenidate hydrochloride) Extended-Release
Capsules – blue capsule
(imprinted with “MLR-02” on cap and “25 mg” on the body)
Contains 25 mg methylphenidate hydrochloride, equivalent to 21.6 mg of methylphenidate -
35 mg (methylphenidate hydrochloride) Extended-Release
Capsules – orange capsule
(imprinted with “MLR-02” on cap and “35 mg” on the body)
Contains 35 mg methylphenidate hydrochloride, equivalent to 30.3 mg of methylphenidate -
45 mg (methylphenidate hydrochloride) Extended-Release
Capsules – yellow capsule
(imprinted with “MLR-02” on cap and “45 mg” on the body)
Contains 45 mg methylphenidate hydrochloride, equivalent to 38.9 mg of methylphenidate -
55 mg (methylphenidate hydrochloride) Extended-Release
Capsules – light green capsule
(imprinted with “MLR-02” on cap and “55 mg” on the body)
Contains 55 mg methylphenidate hydrochloride, equivalent to 47.6 mg of methylphenidate -
70 mg (methylphenidate hydrochloride) Extended-Release
Capsules – iron gray capsule
(imprinted with “MLR-02” on cap and “70 mg” on the body)
Contains 70 mg methylphenidate hydrochloride, equivalent to 60.5 mg of methylphenidate -
85 mg (methylphenidate hydrochloride) Extended-Release
Capsules – white capsule
(imprinted with “MLR-02” on cap and “85 mg” on the body)
Contains 85 mg methylphenidate hydrochloride, equivalent to 73.5 mg of methylphenidate
Contraindications
ADHANSIA XR is contraindicated in patients:
- With a known hypersensitivity to methylphenidate or other components of ADHANSIA XR. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other methylphenidate products [see Adverse Reactions (6.2)].
- Receiving concomitant treatment with monoamine oxidase inhibitors (MAOIs), and also within 14 days following discontinuation of treatment with a MAOI, because of the risk of hypertensive crisis [see Drug Interactions (7.1)].
Warnings and Precautions
Adverse Reactions/Side Effects
The following are discussed in more detail in other sections of the labeling:
- Known hypersensitivity to methylphenidate or other ingredients of ADHANSIA XR [see Contraindications (4)]
- Hypertensive crisis when used concomitantly with monoamine oxidase inhibitors [see Contraindications (4) and Drug Interactions (7.1)]
- Drug dependence [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9.2, 9.3)]
- Serious cardiovascular reactions [see Warnings and Precautions (5.2)]
- Blood pressure and heart rate increases [see Warnings and Precautions (5.3)]
- Psychiatric adverse reactions [see Warnings and Precautions (5.4)]
- Priapism [see Warnings and Precautions (5.5)]
- Peripheral vasculopathy, including Raynaud’s phenomenon [see Warnings and Precautions (5.6)]
- Long-term suppression of growth [see Warnings and Precautions (5.7)]
- Allergic Reactions FD&C Yellow No.5 [see Warnings and Precautions (5.8)]
6.1 Clinical Trial Experience
| Adverse Reaction | ADHANSIA XR | All doses ADHANSIA XR | Placebo | |||
| 25 mg | 45 mg | 70 mg | 100 mg | |||
| N=375 | (N=77) | (N=73) | (N=73) | (N=74) | (N=297) | (N=78) |
| Initial Insomnia | 4% | 8% | 6% | 7% | 6% | 1% |
| Insomnia | 17% | 11% | 16% | 19% | 16% | 4% |
| Dry mouth | 8% | 8% | 7% | 14% | 9% | 4% |
| Nausea | 4% | 6% | 4% | 11% | 6% | 3% |
| Diarrhea | 1% | 3% | 7% | 5% | 4% | 1% |
| Decreased appetite | 4% | 7% | 15% | 19% | 11% | 3% |
| Feeling jittery | 1% | 3% | 8% | 4% | 4% | 1% |
| Weight decreased | 3% | 4% | 3% | 5% | 4% | 1% |
| Upper respiratory tract infection | 0% | 4% | 3% | 3% | 2% | 1% |
| * Includes insomnia, initial insomnia, and delayed sleep phase | |||||||||
| Adverse Reaction | ADHANSIA XR | All doses ADHANSIA XR | Placebo | ||||||
| 25 mg | 35 mg | 45 mg | 55 mg | 70 mg | 85 mg | 100 mg | |||
| N=239 | (N=3) | (N=4) | (N=15) | (N=31) | (N=30) | (N=22) | (N=16) | (N=121) | (N=118) |
| Headache | 0% | 0% | 7% | 0% | 3% | 5% | 13% | 4% | 3% |
| Fatigue | 0% | 0% | 0% | 3% | 3% | 9% | 0% | 3% | 1% |
| Insomnia* | 0% | 0% | 0% | 0% | 7% | 5% | 0% | 3% | 2% |
| Irritability | 0% | 0% | 0% | 0% | 0% | 9% | 0% | 2% | 0% |
| Nausea | 0% | 0% | 7% | 3% | 0% | 0% | 0% | 2% | 0% |
| Dysmenorrhea | 0% | 25% | 0% | 0% | 0% | 5% | 0% | 2% | 0% |
| Adverse Reaction | ADHANSIA XR | All doses ADHANSIA XR | Placebo | |||
| 25 mg | 45 mg | 70 mg | 85 mg | |||
| (N=73) | (N=72) | (N=76) | (N=72) | (N=293) | (N=74) | |
| Decreased appetite | 7% | 19% | 28% | 26% | 20% | 0% |
| Insomnia | 4% | 0% | 9% | 13% | 6% | 1% |
| Initial Insomnia | 4% | 7% | 5% | 4% | 5% | 1% |
| Weight decreased | 1% | 3% | 8% | 13% | 7% | 0% |
| Abdominal pain upper | 5% | 1% | 5% | 4% | 4% | 1% |
| Nausea | 3% | 6% | 7% | 8% | 6% | 4% |
| Dizziness | 3% | 0% | 4% | 4% | 3% | 0% |
| Dry mouth | 1% | 0% | 5% | 4% | 3% | 1% |
| Vomiting | 1% | 1% | 3% | 6% | 3% | 0% |
6.2 Post-marketing Experience
Blood and Lymphatic System Disorders: pancytopenia, thrombocytopenia, thrombocytopenic purpura
Eye Disorders: diplopia, mydriasis, visual impairment
General Disorders: chest pain, chest discomfort, hyperpyrexia
Hepatobiliary disorders: hepatocellular injury, acute hepatic failure
Drug Interactions
7.1 Clinically Important Drug Interactions
Table 3 presents clinically important drug interactions with ADHANSIA XR.
| Monoamine Oxidase Inhibitors (MAOI) | |
| Clinical Impact: | Concomitant use of MAOIs and CNS stimulants can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure [see Contraindications (4)]. |
| Intervention: | Do not administer ADHANSIA XR concomitantly with MAOIs or within 14 days after discontinuing MAOI treatment. |
| Examples: | selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue |
| Gastric pH Modulators | |
| Clinical Impact: | May change the release, PK profiles and alter the pharmacodynamics of ADHANSIA XR. |
| Intervention: | Monitor patients for changes in clinical effect and use alternative therapy based on clinical response. |
| Examples: | Omeprazole, esomeprazole, pantoprazole, famotidine, sodium bicarbonate |
| Antihypertensive Drugs | |
| Clinical Impact: | ADHANSIA XR may decrease the effectiveness of drugs used to treat hypertension [see Warnings and Precautions (5.3)]. |
| Intervention: | Monitor blood pressure and adjust the dosage of the antihypertensive drug as needed. |
| Examples: | Potassium-sparing and thiazide diuretics, calcium channel blockers, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta blockers, centrally acting alpha-2 receptor agonists |
| Risperidone | |
| Clinical Impact: | Combined use of methylphenidate with risperidone when there is a change, whether an increase or decrease, in dosage of either or both medications, may increase the risk of extrapyramidal symptoms (EPS). |
| Intervention: | Monitor for signs of EPS. |
Adhansia XR Description
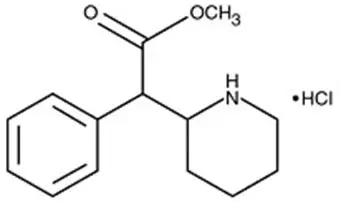
Each strength capsule also contains colorant ingredients in the capsule shell as follows:
- 25 mg FD&C Blue No. 1
- 35 mg FD&C Yellow No. 6, Titanium Dioxide
- 45 mg FD&C Yellow No. 5, Titanium Dioxide
- 55 mg FD&C Blue No. 1, Yellow Iron Oxide, Titanium Dioxide
- 70 mg Black Iron Oxide, Titanium Dioxide
- 85 mg Titanium Dioxide
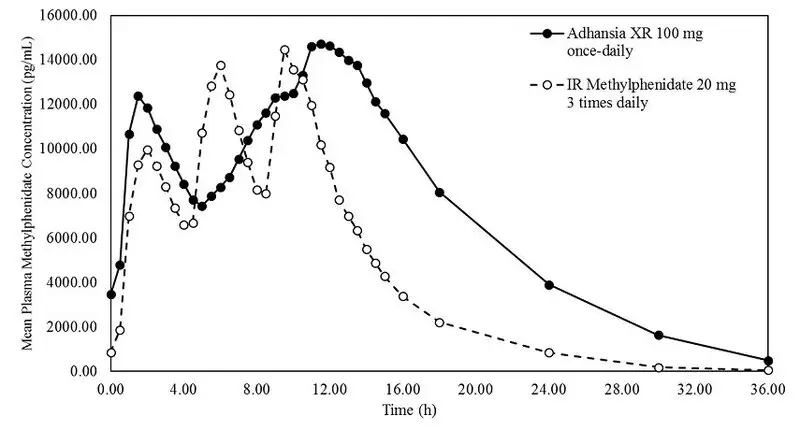
Adhansia XR - Clinical Pharmacology
Clinical Studies
| n: number of subjects included in the primary efficacy
analysis set; SD: standard deviation; SE: standard error; LS Mean:
least-squares mean; CI: confidence interval, not adjusted for multiple
comparisons. a Difference (drug minus placebo) in least-squares mean change from baseline. * Doses that are statistically significantly different from placebo after adjusting for multiplicity. |
|||||
| Study Number | Treatment Group (ADHANSIA XR dose level) | Primary Efficacy Measure: Change from Baseline (Week 1, Visit 2) in ADHD-5-RS Total Score to Week 5 (Visit 6) | |||
| n | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Differencea (95% CI) | ||
| Study 1 | 25 mg | 75 | 36.1 (8.1) | -11.6 (1.31) | -1.9 (-5.6, 1.7) |
| 45 mg* | 73 | 36.5 (7.2) | -16.8 (1.34) | -7.1 (-10.8, -3.4) | |
| 70 mg | 71 | 35.4 (7.4) | -12.0 (1.37) | -2.3 (-6.0, 1.4) | |
| 100 mg* | 72 | 37.0 (7.9) | -17.6 (1.39) | -7.9 (-11.6, -4.1) | |
| Placebo | 77 | 35.7 (8.4) | -9.7 (1.32) | -- | |
| Study 3 | 25 mg | 71 | 37.7 (8.7) | -12.8 (1.35) | -2.2 (-5.9, 1.6) |
| 45 mg* | 68 | 36.4 (8.5) | -16.0 (1.39) | -5.4 (-9.2, -1.6) | |
| 70 mg* | 72 | 35.9 (8.4) | -15.8 (1.35) | -5.2 (-9.0, -1.4) | |
| 85 mg | 70 | 37.8 (8.1) | -15.0 (1.39) | -4.4 (-8.2, -0.6) | |
| Placebo | 71 | 37.3 (8.4) | -10.6 (1.35) | -- | |
| n: number of subjects in the primary efficacy analysis
set; SD: standard deviation; SE: standard error; LS Mean: least-squares
mean; CI: confidence interval, not adjusted for multiple comparisons. a Difference (drug minus placebo) in least-squares mean of post-dose scores. b In each treatment group, three subjects did not have a pre-dose score. Hence, the actual numbers of patients included in the primary analysis were 113 and 110 respectively. |
||||||
| Study Number | Primary Efficacy Measure | Treatment Group | n | Pre-dose Mean Score (SD) | Post-Dose LS Mean Score (SE) | Placebo-subtracted Differencea (95% CI) |
| Study 2 | Average PERMP-T | ADHANSIA XR | 45 | 225.1 (76.7) | 281.3 (4.33) | 26.80 (15.19, 38.41) |
| Placebo | 45 | 235.7 (65.4) | 254.5 (4.63) | -- | ||
| Study 5 | Average PERMP-T | ADHANSIA XR | 116b | 258.2 (89.2) | 302.9 (3.50) | 16.3 (7.6, 24.9) |
| Placebo | 113b | 275.2 (105.8) | 286.6 (3.52) | – | ||
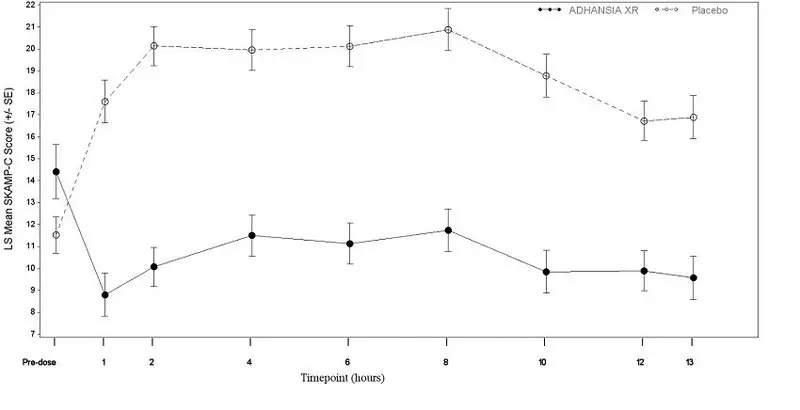
| Study 4 | Average SKAMP-C | ADHANSIA XR | 74 | 14.4 (10.6) | 10.3 (0.74) | -8.6 (-10.6, -6.6) |
| Placebo | 73 | 11.5 (7.1) | 18.9 (0.73) | |||
How is Adhansia XR supplied
ADHANSIA XR® (methylphenidate hydrochloride) extended-release capsules are available as follows:
-
25 mg Capsules – blue, (imprinted
with “MLR-02” and “25 mg”)
Bottles of 30 .....………………………………NDC 72912-525-30
-
35 mg Capsules – orange, (imprinted
with “MLR-02” and “35 mg”)
Bottles of 30 .....………………………………NDC 72912-535-30
-
45 mg Capsules – yellow, (imprinted
with “MLR-02” and “45 mg”)
Bottles of 30 .....………………………………NDC 72912-545-30
-
55 mg Capsules – light green,
(imprinted with “MLR-02” and “55 mg”)
Bottles of 30 .....………………………………NDC 72912-555-30
-
70 mg Capsules – iron gray,
(imprinted with “MLR-02” and “70 mg”)
Bottles of 30 .....………………………………NDC 72912-570-30
-
85 mg Capsules – white, (imprinted
with “MLR-02” and “85 mg”)
Bottles of 30 .....………………………………NDC 72912-585-30
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, including Raynaud’s Phenomenon]
- Instruct patients about the risk of peripheral vasculopathy, including Raynaud’s phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking ADHANSIA XR.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.6)].
Marketed by:
Adlon
Therapeutics L.P.
Stamford, CT 06901-3431
A
subsidiary of Purdue Pharma L.P.
Manufactured by:
Purdue Pharmaceuticals L.P.
Wilson, NC 27893
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised 6/2021 |
| MEDICATION GUIDE ADHANSIA (ad han' see ah) XR® (methylphenidate hydrochloride) extended-release capsules, CII |
|
| What is the
most important information I should know about ADHANSIA XR? ADHANSIA XR can cause serious side effects, including:
|
|
| What is ADHANSIA
XR?
ADHANSIA XR is a central nervous system (CNS) stimulant prescription medicine used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in people 6 years of age and older. ADHANSIA XR may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD. It is not known if ADHANSIA XR is safe and effective in children under 6 years of age. ADHANSIA XR is a federally controlled substance (CII) because it contains methylphenidate that can be a target for people who abuse prescription medicines or street drugs. Keep ADHANSIA XR in a safe place to protect it from theft. Never give your ADHANSIA XR to anyone else, because it may cause death or harm them. Selling or giving away ADHANSIA XR may harm others and is against the law. |
|
Do not take
ADHANSIA XR if you or your child are:
|
|
Before taking
ADHANSIA XR tell your healthcare provider about all medical conditions,
including if you or your child:
ADHANSIA XR and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be changed during treatment with ADHANSIA XR. Your healthcare provider will decide whether ADHANSIA XR can be taken with other medicines. Especially tell your healthcare provider if you or your child take a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI). Know the medicines that you or your child take. Keep a list of the medicines with you to show your healthcare provider and pharmacist. Do not start any new medicine during treatment with ADHANSIA XR without talking to your healthcare provider first. |
|
How should
ADHANSIA XR be taken?
|
|
| What should
be avoided during treatment with ADHANSIA XR?
Avoid drinking alcohol during treatment with ADHANSIA XR. This may cause a faster release of the ADHANSIA XR medicine. |
|
| What are possible
side effects of ADHANSIA XR?
ADHANSIA XR can cause serious side effects, including:
The most common side effects of ADHANSIA XR in children include decreased appetite, trouble sleeping, and decreased weight. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How should
I store ADHANSIA XR?
|
|
| General information
about the safe and effective use of ADHANSIA XR.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ADHANSIA XR for a condition for which it was not prescribed. Do not give ADHANSIA XR to other people, even if they have the same symptoms. It may harm them and it is against the law. You can ask your healthcare provider or pharmacist for information about ADHANSIA XR that was written for healthcare professionals. |
|
| What are the
ingredients in ADHANSIA XR?
Active Ingredient: methylphenidate hydrochloride Inactive Ingredients: ammonio methacrylate copolymer dispersion (type B), anionic copolymer (consisting of methyl acrylate, methyl methacrylate and methacrylic acid), glyceryl monostearate, hypromellose, polyethylene glycol, polysorbate, silicon dioxide, sodium hydroxide, sodium lauryl sulfate, sorbic acid, sugar spheres, triethyl citrate Manufactured by: Purdue Pharmaceuticals L.P., Wilson, NC 27893 To report SUSPECTED ADVERSE REACTIONS, contact Purdue Pharma L.P. at 1-888-726-7535 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. |
|
| ADHANSIA XR
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| ADHANSIA XR
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| ADHANSIA XR
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| ADHANSIA XR
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| ADHANSIA XR
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| ADHANSIA XR
methylphenidate hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Adlon Therapeutics L.P. (116933715) |
| Registrant - Adlon Therapeutics L.P. (116933715) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Glatt Air Techniques | 790220628 | MANUFACTURE(72912-535, 72912-585, 72912-525, 72912-545, 72912-570, 72912-555) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Purdue Pharmaceuticals L.P. | 132080875 | MANUFACTURE(72912-535, 72912-585, 72912-525, 72912-545, 72912-570, 72912-555) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceuticals, Inc. | 080236951 | API MANUFACTURE(72912-525, 72912-535, 72912-585, 72912-555, 72912-570, 72912-545) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Noramco, Inc. | 166506142 | API MANUFACTURE(72912-525, 72912-535, 72912-585, 72912-555, 72912-570, 72912-545) | |