Drug Detail:Adlyxin (Lixisenatide [ lix-i-sen-a-tide ])
Drug Class: Incretin mimetics
Highlights of Prescribing Information
ADLYXIN (lixisenatide) injection, for subcutaneous use
Initial U.S. Approval: 2016
Recent Major Changes
| 06/2022 |
Indications and Usage for Adlyxin
ADLYXIN is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Limitations of Use: (1)
- Has not been studied in patients with chronic pancreatitis or a history of unexplained pancreatitis. Consider other antidiabetic therapies in patients with a history of pancreatitis.
- Not for treatment of type 1 diabetes.
- Has not been studied in patients with gastroparesis and is not recommended in patients with gastroparesis.
Adlyxin Dosage and Administration
- Initiate at 10 mcg once daily for 14 days. On Day 15, increase dosage to 20 mcg once daily. (2.1)
- Administer once daily within one hour before the first meal of the day. (2.2)
- Inject subcutaneously in the abdomen, thigh or upper arm. (2.2)
Dosage Forms and Strengths
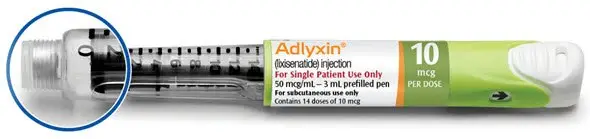
- Injection: 50 mcg/mL in a 3 mL single-patient-use prefilled pen (for 14 doses of 10 mcg per dose). (3)
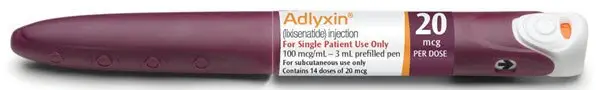
- Injection: 100 mcg/mL in a 3 mL single-patient-use prefilled pen (for 14 doses of 20 mcg per dose). (3)
Contraindications
Severe hypersensitivity to lixisenatide or any component of ADLYXIN. (4)
Warnings and Precautions
- Anaphylaxis and Serious Hypersensitivity Reactions: Discontinue ADLYXIN and promptly seek medical advice. (5.1)
- Pancreatitis: Discontinue promptly if pancreatitis is suspected. Do not restart if pancreatitis is confirmed. Consider other antidiabetic therapies in patients with a history of pancreatitis. (5.2)
- Never share ADLYXIN pen between patients, even if the needle is changed. (5.3)
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Patients taking an insulin secretagogue or insulin may have an increased risk of hypoglycemia, including severe hypoglycemia. Reduction in the dose of insulin secretagogues or insulin may be necessary. (5.4)
- Acute Kidney Injury: Monitor renal function in patients with renal impairment reporting severe adverse gastrointestinal reactions. ADLYXIN is not recommended in patients with end stage renal disease. (5.5)
- Immunogenicity: Patients may develop antibodies to lixisenatide. If there is worsening glycemic control or failure to achieve targeted glycemic control, significant injection site reactions or allergic reactions, alternative antidiabetic therapy should be considered. (5.6)
- Acute Gallbladder Disease: If cholelithiasis or cholecystitis are suspected, gallbladder studies are indicated. (5.7)
Adverse Reactions/Side Effects
The most common adverse reactions (≥5%) of patients treated with ADLYXIN are nausea, vomiting, headache, diarrhea, dizziness, and hypoglycemia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- ADLYXIN delays gastric emptying which may impact absorption of concomitantly administered oral medications. Oral medications that are particularly dependent on threshold concentrations for efficacy, such as antibiotics, or medications for which a delay in effect is undesirable, such as acetaminophen, should be administered 1 hour before ADLYXIN. (7.1, 12.3)
- Oral contraceptives should be taken at least 1 hour before ADLYXIN administration or 11 hours after the dose of ADLYXIN. (7.1, 12.3)
Use In Specific Populations
Pregnancy: ADLYXIN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2022
Full Prescribing Information
1. Indications and Usage for Adlyxin
ADLYXIN is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2. Adlyxin Dosage and Administration
2.1 Dosing Instructions
- The starting dose of ADLYXIN is 10 mcg subcutaneously once daily for 14 days.
- Increase the dose to the maintenance dose of 20 mcg once daily starting on Day 15.
2.2 Important Administration Instructions
- Instruct patients and caregivers on the preparation and use of the pen prior to first use of ADLYXIN. Training should include a practice injection.
- Inspect ADLYXIN visually before use. It should appear clear and colorless. Do not use ADLYXIN if particulate matter or coloration is seen.
- Administer ADLYXIN by subcutaneous injection in the abdomen, thigh or upper arm once daily.
- Rotate injections sites with each dose. Do not use the same site for each injection.
- Instruct patients to administer an injection of ADLYXIN within one hour before the first meal of the day preferably the same meal each day. If a dose is missed, administer ADLYXIN within one hour prior to the next meal.
3. Dosage Forms and Strengths
ADLYXIN is a clear and colorless solution available as:
Injection: 50 mcg/mL in 3 mL solution in a green single-patient-use prefilled pen (for 14 doses of 10 mcg per dose)
Injection: 100 mcg/mL in 3 mL solution in a burgundy single-patient-use prefilled pen (for 14 doses of 20 mcg per dose)
4. Contraindications
ADLYXIN is contraindicated in patients with known severe hypersensitivity to lixisenatide or to any component of ADLYXIN. Hypersensitivity reactions including anaphylaxis have occurred with ADLYXIN [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
5. Warnings and Precautions
5.1 Anaphylaxis and Serious Hypersensitivity Reactions
In clinical trials of ADLYXIN, there have been cases of anaphylaxis determined to be related to ADLYXIN (frequency of 0.1% or 10 cases per 10,000 patient-years). Other serious hypersensitivity reactions including angioedema also occurred [see Adverse Reactions (6.1)].
Inform and closely monitor patients with a history of anaphylaxis or angioedema with another GLP-1 receptor agonist for allergic reactions, because it is unknown whether such patients will be predisposed to anaphylaxis with ADLYXIN. ADLYXIN is contraindicated in patients with known hypersensitivity to lixisenatide [see Contraindications (4)]. If a hypersensitivity reaction occurs, the patient should discontinue ADLYXIN and promptly seek medical attention.
5.2 Pancreatitis
Acute pancreatitis, including fatal and nonfatal hemorrhagic or necrotizing pancreatitis, has been reported postmarketing in patients treated with GLP-1 receptor agonists. In clinical trials of ADLYXIN, there were 21 cases of pancreatitis among ADLYXIN-treated patients and 14 cases in comparator-treated patients (incidence rate of 21 vs. 17 per 10,000 patient-years). ADLYXIN cases were reported as acute pancreatitis (n=3), pancreatitis (n=12), chronic pancreatitis (n=5), and edematous pancreatitis (n=1). Some patients had risk factors for pancreatitis, such as a history of cholelithiasis or alcohol abuse.
After initiation of ADLYXIN, observe patients carefully for signs and symptoms of pancreatitis (including persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting). If pancreatitis is suspected, promptly discontinue ADLYXIN and initiate appropriate management. If pancreatitis is confirmed, do not restart ADLYXIN. Consider antidiabetic therapies other than ADLYXIN in patients with a history of pancreatitis.
5.3 Never Share ADLYXIN Pen Between Patients
ADLYXIN pens should never be shared between patients, even if the needle is changed. Pen-sharing poses a risk for transmission of blood-borne pathogens.
5.4 Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin
Patients receiving ADLYXIN in combination with basal insulin or insulin secretagogue (e.g., sulfonylurea) have an increased risk of hypoglycemia, including severe hypoglycemia. In patients receiving sulfonylurea with or without metformin, 14.5% patients on ADLYXIN reported symptomatic hypoglycemia compared to 10.6% for those on placebo. In patients receiving basal insulin with or without metformin, 28.3% patients on ADLYXIN reported symptomatic hypoglycemia compared to 23.0% for those on placebo. In patients receiving basal insulin with sulfonylurea, 47.2% patients on ADLYXIN reported symptomatic hypoglycemia compared to 21.6% for those on placebo [see Adverse Reactions (6.1)].
The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia [see Drug Interactions (7.2)].
5.5 Acute Kidney Injury
Acute kidney injury and worsening of chronic renal failure, which may sometimes require hemodialysis has been reported postmarketing in patients treated with ADLYXIN. Some of these events were reported in patients without known underlying renal disease. A majority of the reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration.
Monitor renal function when initiating or escalating doses of ADLYXIN in patients with renal impairment and in patients reporting severe gastrointestinal reactions. ADLYXIN is not recommended in patients with end stage renal disease [see Use in Specific Populations (8.6)].
5.6 Immunogenicity
Patients may develop antibodies to lixisenatide following treatment with ADLYXIN. A pooled analysis of studies of lixisenatide-treated patients showed that 70% were antibody positive at Week 24. In the subset of patients (2.4 %) with the highest antibody concentrations (>100 nmol/L), an attenuated glycemic response was observed. A higher incidence of allergic reactions and injection site reactions occurred in antibody positive patients [see Warnings and Precautions (5.1), Adverse Reactions (6.2)].
If there is worsening glycemic control or failure to achieve targeted glycemic control, significant injection site reactions or allergic reactions, alternative antidiabetic therapy should be considered [see Adverse Reactions (6.1)].
5.7 Acute Gallbladder Disease
Acute events of gallbladder disease such as cholelithiasis or cholecystitis have been reported in GLP-1 receptor agonist trials and postmarketing. In the ELIXA study [see Clinical Studies (14.6)], cholelithiasis occurred in 0.4% of ADLYXIN-treated patients versus 0.2% in placebo-treated patients and acute cholecystitis in 0.3% of ADLYXIN-treated patients versus 0.2% in placebo-treated patients. If cholelithiasis is suspected, gallbladder studies and appropriate clinical follow-up are indicated.
6. Adverse Reactions/Side Effects
The following serious reactions are described below or elsewhere in the prescribing information:
- Anaphylaxis and Serious Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Pancreatitis [see Warnings and Precautions (5.2)]
- Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin [see Warnings and Precautions (5.4)]
- Renal Failure [see Warnings and Precautions (5.5)]
- Immunogenicity [see Warnings and Precautions (5.6)]
- Acute Gallbladder Disease [see Warnings and Precautions 5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pool of Placebo-Controlled Trials
The data in Table 1 are derived from the placebo-controlled trials [see Clinical Studies (14)].
These data reflect exposure of 2869 patients to ADLYXIN and a mean duration of exposure to ADLYXIN of 21.7 weeks. Across the treatment arms, the mean age of patients was 56.1 years, 2.3% were 75 years or older and 48.2% were male. The population in these studies was 63.7% White, 2.6% Black or African American, 32.0% Asian, and 18.9% were of Hispanic or Latino ethnicity. At baseline, the population had diabetes for an average of 8.2 years and had a mean HbA1c of 8.1%. At baseline, 11.2% of the population reported retinopathy. Baseline estimated renal function was normal or mildly impaired (eGFR ≥60 mL/min/1.73 m2) in 95.3% of the pooled study populations.
Table 1 shows common adverse reactions, excluding hypoglycemia, associated with the use of ADLYXIN in the pool of placebo-controlled trials. These adverse reactions were not present at baseline, occurred more commonly on ADLYXIN than on placebo, and occurred in at least 5% of patients treated with ADLYXIN.
| Adverse Reaction | Placebo (N=1639) | ADLYXIN (N=2869) |
|---|---|---|
| * hypoglycemia is discussed separately. | ||
| Nausea | 6% | 25% |
| Vomiting | 2% | 10% |
| Headache | 6% | 9% |
| Diarrhea | 6% | 8% |
| Dizziness | 4% | 7% |
Hypoglycemia
Symptomatic hypoglycemia was defined as an event with clinical symptoms that were considered to result from a hypoglycemic episode with an accompanying plasma glucose <60 mg/dL or associated with prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration if no plasma glucose value was available.
Severe symptomatic hypoglycemia was defined as an event with clinical symptoms that were considered to result from hypoglycemia in which the patient required the assistance of another person, associated with a plasma glucose level below 36 mg/dL or, associated with prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration if no plasma glucose was available.
Table 2 summarizes the incidence of symptomatic hypoglycemia and severe hypoglycemia in seven placebo-controlled efficacy/safety studies.
| Background Therapy | Placebo | ADLYXIN |
|---|---|---|
|
||
| Monotherapy* | N=122 | N=239 |
| Symptomatic (%) | 2 | 2 |
| Severe (%) | 0 | 0 |
| With Metformin | N=432 | N=946 |
| Symptomatic (%) | 1 | 3 |
| Severe (%) | 0 | 0 |
| With Sulfonylurea +/- metformin | N=377 | N=656 |
| Symptomatic (%) | 11 | 15 |
| Severe (%) | 0 | 0.2 |
| With Pioglitazone +/- metformin | N=161 | N=323 |
| Symptomatic (%) | 1 | 3 |
| Severe (%) | 0 | 0 |
| With Basal insulin +/- metformin | N=213 | N=374 |
| Symptomatic (%) | 23 | 28 |
| Severe (%) | 0 | 1 |
| With Basal insulin +/- sulfonylurea | N=111 | N=108 |
| Symptomatic (%) | 22 | 47 |
| Severe (%) | 0 | 0 |
| With Insulin Glargine and metformin +/- thiazolidinedione | N=223 | N=223 |
| Symptomatic (%) | 14 | 22 |
| Severe (%) | 0 | 0.4 |
6.2 Immunogenicity
In the pool of 9 placebo-controlled studies, 70% of patients exposed to lixisenatide tested positive for anti-lixisenatide antibodies during the trials. In the subset of patients (2.4%) with the highest antibody concentrations (>100 nmol/L), an attenuated glycemic response was observed. A higher incidence of allergic reactions and injection site reactions occurred in antibody positive patients [see Warnings and Precautions (5.6)].
Anti-lixisenatide antibody characterization studies have demonstrated the potential for development of antibodies cross-reactive with endogenous GLP-1 and glucagon, but the clinical significance of these antibodies is not currently known.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, the incidence of antibodies to lixisenatide cannot be directly compared with the incidence of antibodies with other products.
6.3 Post-Marketing Experience
The following additional adverse reactions have been reported during post-approval use of ADLYXIN. Because these events are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary: cholecystitis, cholelithiasis requiring cholecystectomy
7. Drug Interactions
7.1 Delayed Gastric Emptying Effects on Oral Medications
ADLYXIN delays gastric emptying which may reduce the rate of absorption of orally administered medications. Use caution when coadministering oral medications that have a narrow therapeutic ratio or that require careful clinical monitoring. These medications should be adequately monitored when concomitantly administered with ADLYXIN. If such medications are to be administered with food, patients should be advised to take them with a meal or snack when ADLYXIN is not administered.
Oral medications that are particularly dependent on threshold concentrations for efficacy, such as antibiotics, or medications for which a delay in effect is undesirable, such as acetaminophen, should be administered at least 1 hour before ADLYXIN injection [see Clinical Pharmacology (12.3)].
Patients taking oral contraceptives should be advised to take them at least 1 hour before ADLYXIN administration or at least 11 hours after the dose of ADLYXIN [see Clinical Pharmacology (12.3)].
7.2 Dosage Adjustment of Sulfonylurea or Insulin with Concomitant Use with ADLYXIN
When initiating ADLYXIN, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)].
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of ADLYXIN have not been established in pediatric patients.
8.5 Geriatric Use
In clinical studies of ADLYXIN, a total of 1837 (25%) of the patients exposed to the study medication were 65 years of age and over and 288 (4%) were 75 years of age and over. No overall differences were observed in safety or effectiveness between these patients and younger patients, but individual sensitivity cannot be ruled out.
8.6 Renal Impairment
In patients with mild renal impairment (eGFR: 60–89 mL/min/1.73 m2) no dose adjustment is required [see Clinical Pharmacology (12.3)] but close monitoring for ADLYXIN related adverse reactions [see Adverse Reactions (6.1)] and for changes in renal function is recommended because a higher incidence of hypoglycemia, nausea and vomiting were observed in these patients.
In a cardiovascular outcome study, 655 (22%) lixisenatide treated patients had moderate renal impairment (eGFR: 30 to less than 60 mL/min/1.73 m2). No dosing adjustment is recommended in patients with moderate renal impairment [see Clinical Pharmacology (12.3)] but close monitoring for ADLYXIN related adverse gastrointestinal reactions [see Adverse Reactions (6.1)] and for changes in renal function is recommended because these may lead to dehydration and acute renal failure and worsening of chronic failure in these patients [see Warnings and Precautions (5.5)].
Clinical experience in patients with severe renal impairment is limited as there were only 5 patients with severe renal impairment (eGFR 15 to less than 30 mL/min/1.73 m2) exposed to ADLYXIN in all controlled studies. Lixisenatide exposure was higher in these patients [see Clinical Pharmacology (12.3)]. Patients with severe renal impairment exposed to ADLYXIN should be closely monitored for occurrence of gastrointestinal adverse reactions and for changes in renal function [see Warnings and Precautions (5.5)].
There is no therapeutic experience in patients with end stage renal disease (eGFR <15 mL/min/1.73 m2), and it is not recommended to use ADLYXIN in this population [see Clinical Pharmacology (12.3)].
10. Overdosage
In case of overdose, appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms.
11. Adlyxin Description
Lixisenatide is a synthetic analogue of human GLP-1 and acts as a GLP-1 receptor agonist. Lixisenatide is a protein containing 44 amino acids, which is amidated at the C-terminal amino acid (position 44) and has a molecular weight of 4.8585 kDa.
ADLYXIN (lixisenatide) injection is a sterile, clear and colorless solution for subcutaneous use. ADLYXIN is supplied as 3 mL single-patient-use prefilled pens. Each mL contains either 50 mcg of lixisenatide (green prefilled pen) or 100 mcg of lixisenatide (burgundy prefilled pen) and the inactive ingredients: glycerol 85% (54 mg), metacresol (8.1 mg), methionine (9.0 mg), sodium acetate trihydrate (10.5 mg), and Water for Injection, USP. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH. The pH is approximately 4.5.
12. Adlyxin - Clinical Pharmacology
12.1 Mechanism of Action
Lixisenatide is a GLP-1 receptor agonist. Lixisenatide increases glucose-dependent insulin release, decreases glucagon secretion, and slows gastric emptying.
12.2 Pharmacodynamics
In a clinical pharmacology study in adults with type 2 diabetes mellitus, ADLYXIN reduced fasting plasma glucose and postprandial blood glucose AUC0–300min compared to placebo (-33.8 mg/dL and -387 mg∙h/dL, respectively) following a standardized test meal. The effect on postprandial blood glucose AUC was most notable with the first meal, and the effect was attenuated with later meals in the day.
14. Clinical Studies
ADLYXIN has been studied as monotherapy, in combination with oral antidiabetic medications, and in combination with basal insulin (with or without oral antidiabetic medications). The efficacy of ADLYXIN was compared with placebo, exenatide, and insulin glulisine.
In patients with type 2 diabetes, ADLYXIN produced reductions from baseline in HbA1c compared to placebo.
14.1 Monotherapy
In a 12-week double blind study, 241 patients with type 2 diabetes inadequately controlled on diet and exercise were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 53.9 years, and the mean duration of type 2 diabetes mellitus was 2.5 years; 51.0% were male, 72.6% were White, 2.5% were Black or African American, 22.0% were Hispanic, and 3.7% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 32 kg/m2.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 12 (see Table 3). The adjusted mean change in weight from baseline did not differ significantly between ADLYXIN (-1.9 kg) and placebo (-2.0 kg).
| Placebo (N=122) | ADLYXIN 20 mcg (N=119) |
|
|---|---|---|
| ITT population = all randomized patients. | ||
| 10% of patients in ADLYXIN and 10% in the placebo had missing HbA1c data at Week 12 in the ITT population. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 8.07 | 8.07 |
| LS mean change from baseline* | -0.18 | -0.83 |
| Difference from placebo (95% CI) | -0.65 (-0.903, -0.399) (p<0.0001) |
|
| Patients (%) achieving HbA1c <7.0%† | 24 | 44 |
| Fasting plasma glucose (FPG) (mg/dL) | ||
| Baseline (mean) | 160.39 | 162.77 |
| LS mean change from baseline* | 1.46 | -15.84 |
| Body weight (kg) | ||
| Baseline (mean) | 86.08 | 86.50 |
| LS mean change from baseline* | -2.03 | -1.94 |
14.2 Add-on Combination Therapy to Metformin (Alone or in Combination with Sulfonylurea)
In a 24-week study, 323 patients with type 2 diabetes inadequately controlled on diet, exercise, and metformin were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 56.7 years, and the mean duration of type 2 diabetes mellitus was 5.9 years; 44.6% were male, 90.1% were White, 0.6% were Black or African American, 27.9% were Hispanic, and 1.2% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 33 kg/m2. The mean dose of metformin was 1955 mg per day.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 24 (see Table 4).
| Background Therapy | with Metformin* | |
|---|---|---|
| Placebo (N=162) | ADLYXIN 20 mcg (N=161) |
|
| ITT population = all randomized patients. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 8.03 | 7.99 |
| LS mean change from baseline† | -0.26 | -0.72 |
| Difference from placebo (95% CI) | -0.46 (-0.640, -0.279) (p<0.0001) |
|
| Patients (%) achieving HbA1c <7.0%‡ | 22 | 44 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) | 170.32 | 172.23 |
| LS mean change from baseline† | -7.25 | -16.88 |
| Difference from placebo (95% CI) | -9.64 (-16.306, -2.970) (p=0.0046) |
|
| Body weight (kg) | ||
| Baseline (mean) | 87.87 | 90.21 |
| LS mean change from baseline† | -1.71 | -2.70 |
| Difference from placebo (95% CI) | -1.00 (-1.706, -0.286) (p=0.006) |
|
In a 24-week study, 391 Asian patients with type 2 diabetes inadequately controlled on diet, exercise, and metformin with or without a sulfonylurea were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 54.8 years, and mean duration of type 2 diabetes mellitus was 6.6 years. 49.1% were male. All patients were Asian. 2.8% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 27 kg/m2. The mean dose of metformin was 1368 mg per day and 44.8% of patients were on a sulfonylurea.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 24 (see Table 5).
| Background Therapy | with Metformin +/- Sulfonylurea* | |
|---|---|---|
| Placebo (N=195) | ADLYXIN 20 mcg (N=196) |
|
| ITT population = all randomized patients. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 7.85 | 7.95 |
| LS mean change from baseline† | -0.57 | -0.84 |
| Difference from placebo (95% CI) | -0.27 (-0.447, -0.090) (p=0.0032) |
|
| Patients (%) achieving HbA1c <7.0%‡ | 37 | 49 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) | 157.47 | 159.26 |
| LS mean change from baseline† | -7.05 | -13.39 |
| Body weight (kg) | ||
| Baseline (mean) | 72.74 | 73.18 |
| LS mean change from baseline† | -1.12 | -1.36 |
In a 24-week open-label study, 634 patients with type 2 diabetes inadequately controlled on diet, exercise and metformin were randomized to ADLYXIN 20 mcg once daily or exenatide 10 mcg twice daily. The mean age of the study population was 57.4 years, and mean duration of type 2 diabetes mellitus was 6.8 year; 53.3% were male, 92.7% were White, 2.8% were Black or African American, 26.8% were Hispanic, and 1.7% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 34 kg/m2. The mean dose of metformin was 2039 mg per day.
ADLYXIN 20 mcg once daily met the prespecified noninferiority margin of 0.4% versus exenatide 10 mg BID for the difference in HbA1c reduction from baseline (see Table 6). However in this study, ADLYXIN provided less HbA1c reduction than exenatide 10 mg BID and the difference was statistically significant (p=0.0175).
| ADLYXIN (N=318) | Exenatide BID (N=316) |
|
|---|---|---|
| ITT population = all randomized patients. | ||
| 14% of patients in ADLYXIN and 14% in exenatide had missing HbA1c data at Week 24 in the ITT population. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 7.95 | 7.97 |
| LS Mean change from baseline* | -0.73 | -0.90 |
| LS mean difference vs Exenatide BID* | 0.17 | |
| 95% CI | (0.030 to 0.314) (p=0.0175) | |
| Patients (%) achieving HbA1c <7.0%† | 43.1 | 45.6 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline | 174.24 | 173.88 |
| LS Mean change from baseline | -19.79 | -24.19 |
| Body weight (kg) | ||
| Baseline | 94.01 | 96.09 |
| LS Mean change from baseline | -2.74 | -3.72 |
14.3 Add-on Combination Therapy to a Sulfonylurea (Alone or in Combination with Metformin)
In a 24-week study, 859 patients with type 2 diabetes inadequately controlled with diet, exercise and a sulfonylurea with or without metformin were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 57.2 years, and mean duration of type 2 diabetes mellitus was 9.3 years; 50.5% were male, 52.2% were White, 3.0% were Black or African American, 2.7% were Hispanic, and 4.7% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 30 kg/m2. The two most common sulfonylureas used were glimepiride and glibenclamide and the mean dose of these drugs at baseline were 5.1 mg and 12.9 mg, respectively, and 84.4% of patients were on metformin.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 24 (see Table 7).
| Background Therapy | with Sulfonylurea +/- Metformin* | |
|---|---|---|
| Placebo (N=286) | ADLYXIN 20 mcg (N=573) |
|
| ITT population = all randomized patients. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 8.21 | 8.28 |
| LS mean change from baseline† | -0.18 | -0.77 |
| Difference from placebo (95% CI) | -0.58 (-0.715, -0.453) (p<0.0001) |
|
| Patients (%) achieving HbA1c <7.0%‡ | 13 | 33 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) | 167.47 | 174.24 |
| LS mean change from baseline† | -10.36 | -17.09 |
| Difference from placebo (95% CI) | -6.73 (-11.946, -1.518) (p=0.0114) |
|
| Body weight (kg) | ||
| Baseline (mean) | 84.34 | 82.34 |
| LS mean change from baseline† | -0.83 | -1.63 |
| Difference from placebo (95% CI) | -0.80 (-1.244, -0.349) (p=0.0005) |
|
14.4 Add-on Treatment to Pioglitazone (Alone or in Combination with Metformin)
In a 24-week study, 484 patients with type 2 diabetes with inadequately controlled with diet, exercise and pioglitazone with or without metformin were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 55.8 years, and mean duration of type 2 diabetes mellitus was 8.1 years; 52.5% were male, 83.7% were White, 4.8% were Black or African American, 26.4% were Hispanic, and 4.1% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 34 kg/m2. The mean dose of pioglitazone was 33.6 mg per day and 81.0% of patients were on metformin.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 24 (see Table 8).
| Background Therapy | Pioglitazone +/- Metformin* | |
|---|---|---|
| Placebo (N=161) | ADLYXIN 20 mcg (N=323) |
|
| ITT population = all randomized patients. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 8.06 | 8.08 |
| LS mean change from baseline† | -0.43 | -0.91 |
| Difference from placebo (95% CI) | -0.48 (-0.647, -0.318) (p<0.0001) |
|
| Patients (%) achieving HbA1c <7.0%‡ | 25 | 49 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) | 164.49 | 164.16 |
| LS mean change from baseline† | -14.12 | -24.56 |
| Difference from placebo (95% CI) | -10.45 (-16.580, -4.315) (p=0.0008) |
|
| Body weight (kg) | ||
| Baseline (mean) | 96.74 | 92.93 |
| LS mean change from baseline† | 0.26 | -0.11 |
14.5 Add-on to Basal Insulin (Alone or in Combination with Oral Antidiabetics)
In a 24-week study, 496 patients with type 2 diabetes inadequately controlled on diet, exercise and basal insulin with or without metformin were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 57.2 years, and mean duration of type 2 diabetes mellitus was 12.46 years; 46.0% were male, 77.6% were White, 4.0% were Black or African American, 27.0% were Hispanic, and 3.2% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 32 kg/m2. At baseline, the mean basal insulin dose was 54.9 units and 79.2% of individuals were receiving metformin.
In another 24-week study, 311 Asian patients with type 2 diabetes inadequately controlled on diet, exercise and basal insulin with or without a sulfonylurea were randomized to ADLYXIN 20 mcg once daily or to placebo. The mean age of the study population was 58.4 years, and mean duration of type 2 diabetes mellitus was 13.92 years; 47.9% were male, all patients were Asian, and 15.8% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 25 kg/m2. At baseline, the mean basal insulin dose was 24.2 units and 70.4% of individuals were receiving a sulfonylurea.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 24 (see Table 9) in both studies.
| Background Therapy | with Basal Insulin +/- Metformin* | with Basal Insulin +/- Sulfonylurea† | ||
|---|---|---|---|---|
| Placebo (N=167) | ADLYXIN 20 mcg (N=329) | Placebo (N=157) | ADLYXIN 20 mcg (N=154) |
|
| ITT population = all randomized patients. | ||||
|
||||
| HbA1c (%) | ||||
| Baseline (mean) | 8.37 | 8.42 | 8.52 | 8.54 |
| LS mean change from baseline‡ | -0.34 | -0.71 | 0.07 | -0.70 |
| Difference from placebo (95% CI) | -0.36 (-0.557, -0.170) (p=0.0002) | -0.76 (-1.005, -0.516) (p<0.0001) |
||
| Patients (%) achieving HbA1c <7.0%§ | 11 | 25 | 6 | 33 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||||
| Baseline (mean) | 144.94 | 146.44 | 139.69 | 138.25 |
| LS mean change from baseline‡ | -13.07 | -13.02 | 2.02 | -4.38 |
| Body weight (kg) | ||||
| Baseline (mean) | 88.94 | 87.10 | 65.60 | 65.93 |
| LS mean change from baseline‡ | -0.36 | -1.55 | -0.03 | -0.48 |
In a 24-week study, 446 patients with type 2 diabetes, inadequately controlled on diet exercise and on insulin glargine and metformin with or without thiazolidinediones, were randomized to ADLYXIN 20 mcg once daily or placebo. The mean age of the study population was 56.2 years, and mean duration of type 2 diabetes mellitus was 9.1 years; 49.8% were male, 74.4% were White, 4.5% were Black or African American, 22.6% were Hispanic, and 3.8% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 32 kg/m2. At baseline, the mean insulin glargine dose was 44.5 units, the mean metformin dose was 2049 mg and 12.1% of individuals were receiving thiazolidinedione.
Compared with placebo, treatment with ADLYXIN 20 mcg once daily resulted in statistically significant reductions in HbA1c from baseline at Week 24 (see Table 10).
| Background Therapy | with Insulin Glargine and Metformin +/- Thiazolidinediones* | |
|---|---|---|
| Placebo (N=223) | ADLYXIN 20 mcg (N=223) |
|
| ITT population = all randomized patients. | ||
|
||
| HbA1c (%) | ||
| Baseline (mean) | 7.60 | 7.56 |
| LS mean change from baseline† | -0.42 | -0.70 |
| Difference from placebo (95% CI) | -0.28 (-0.434, -0.123) (p=0.0005) |
|
| Patients (%) achieving HbA1c <7.0%‡ | 39 | 50 |
| Fasting Plasma Glucose (FPG) (mg/dL) | ||
| Baseline (mean) | 120.67 | 117.99 |
| LS mean change from baseline† | 6.05 | 5.74 |
| Body weight (kg) | ||
| Baseline (mean) | 86.75 | 87.31 |
| LS mean change from baseline† | 1.09 | 0.31 |
| Difference from placebo (95% CI) | -0.78 (-1.388, -0.168) (p=0.0125) |
|
In a 26-week open-label study, 894 patients with type 2 diabetes inadequately controlled on diet, exercise and basal insulin combined with 1 to 3 oral antidiabetic agents were randomized to ADLYXIN 20 mcg once daily or insulin glulisine once daily (QD) or insulin glulisine three times a day (TID) combined with insulin glargine with or without metformin. The mean age of the study population was 59.8 years, and mean duration of type 2 diabetes mellitus was 12.2 years; 45.3% were male, 92.6% were White, 4.0% were Black or African American, 21.1% were Hispanic, and 5.6% had an eGFR <60 mL/min/1.73 m2. The mean BMI was 32 kg/m2. At baseline, the mean insulin glargine dose was 65.9 units and 87.4% of individuals were receiving metformin.
ADLYXIN 20 mcg once daily met the prespecified noninferiority margin of 0.4% versus insulin glulisine QD and TID for the difference in HbA1c reduction from baseline. However in this study, ADLYXIN provided less HbA1c reduction than insulin glulisine TID and the difference was statistically significant (p=0.0002).
| ADLYXIN | Insulin Glulisine QD | Insulin Glulisine TID | |
|---|---|---|---|
| (N=298) | (N=298) | (N=298) | |
| ITT population = all randomized patients. Noninferiority margin = 0.4%. | |||
| 12% of patients in ADLYXIN, 8% in glulisine QD and 5.0% in glulisine TID had missing HbA1c data at Week 26 in the ITT population. | |||
|
|||
| HbA1c (%) | |||
| Baseline | 7.77 | 7.73 | 7.79 |
| LS Mean change from baseline* | -0.57 | -0.53 | -0.80 |
| LS mean difference vs insulin glulisine* | -0.04 | 0.23 | |
| 95% CI | (-0.161 to 0.080) | (0.112 to 0.352) (p=0.0002) |
|
| Patients (%) achieving HbA1c <7.0%† | 38.6 | 36.6 | 47.7 |
| Fasting Plasma Glucose (FPG) (mg/dL) | |||
| Baseline | 118.55 | 123.21 | 119.80 |
| LS Mean change from baseline* | -3.39 | -3.68 | -1.42 |
| Body weight (kg) | |||
| Baseline | 90.06 | 88.45 | 90.08 |
| LS Mean change from baseline* | -0.64 | 0.98 | 1.26 |
| LS mean difference vs insulin glulisine* | -1.91 | ||
| 95% CI | (-3.103 to -0.713) (p=0.0018) |
||
14.6 ELIXA Cardiovascular Outcome Study
The ELIXA study was a randomized, double-blind, placebo-controlled, multinational study that evaluated cardiovascular (CV) outcomes during treatment with ADLYXIN in patients with type 2 diabetes mellitus after a recent Acute Coronary Syndrome event. The primary composite endpoint was the time to the first occurrence of a major adverse cardiovascular event or MACE+, defined as any of the following positively adjudicated events: Cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, or hospitalization for unstable angina. The study was designed as a noninferiority trial with a prespecified risk margin of 1.3 for the hazard ratio comparing ADLYXIN to placebo.
Overall, 6068 patients were randomized 1:1 to either placebo or ADLYXIN 20 mcg (following a starting dose of 10 mcg during the first 2 weeks) and were included in the primary analyses. The demographics and baseline characteristics were well-balanced between treatments. The median age at study entry was 60 years. Approximately 69% of the patients were males and 75% were Caucasian. The majority of patients were either obese or overweight with a median BMI of 29.4 kg/m2. The mean duration of diabetes was 9.3 years. More than 75% of patients had impaired renal function and more than 20% had an estimated GFR less than 60 mL/min/ 1.73 m2. Use of CV medications at baseline was similar between treatments; platelet aggregation inhibitors (aspirin and/or clopidogrel) were used by 97.5% of patients, statins by 92.7%, ACE inhibitors and/or angiotensin II antagonists by 86.8%, and beta-blockers by 84.4%. Prior to study entry, 93.9% of patients used at least 1 glucose-lowering medication, including metformin (69.9%), sulfonylureas (37.3%) and insulin (47.6%). During the study, antidiabetic medications were adjusted by the investigators per the local standard of care.
Ninety-six percent of the patients in both treatment groups completed the study in accordance with the protocol, and the vital status was known at the end of the study for 99.0% and 98.6% of the patients in the ADLYXIN and placebo group, respectively. Median treatment duration was 22.4 months in the ADLYXIN group and 23.3 months in the placebo group, and the median duration of study follow-up was 25.8 and 25.7 months, respectively.
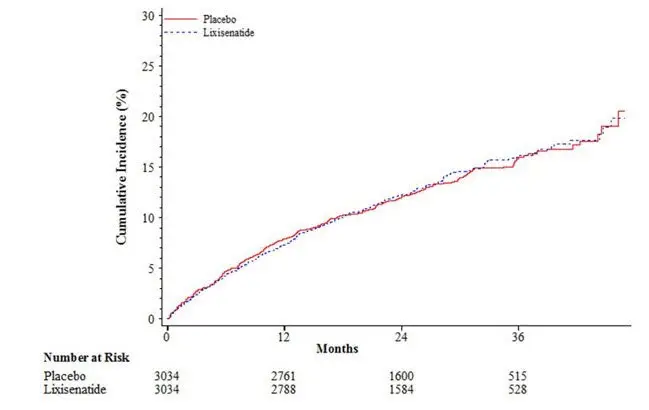
The results of the primary composite cardiovascular endpoint are shown in Table 12. The hazard ratio (HR) for ADLYXIN versus placebo was 1.02, with an associated 2-sided 95% confidence interval (CI) of 0.89 to 1.17. The upper bound of this confidence interval, 1.17, excluded a risk margin larger than 1.3.
| Placebo (N=3,034) | ADLYXIN (N=3,034) | Hazard ratio (95% CI) |
|
|---|---|---|---|
| CI: confidence interval, CV: cardiovascular. Only positively adjudicated events by the Cardiovascular Events Adjudication Committee are included. |
|||
| Primary composite CV event | 1.02 (0.89, 1.17) |
||
| No. of patients with event (%) | 399 (13.2%) | 406 (13.4%) | |
| Total Person Year | 6328.2 | 6356.8 | |
| Incidence Rate | 6.31 | 6.39 | |
| Component CV event | |||
| Cardiovascular death | 93 (3.1%) | 88 (2.9%) | |
| Nonfatal myocardial infarction | 247 (8.1%) | 255 (8.4%) | |
| Nonfatal stroke | 49 (1.6%) | 54 (1.8%) | |
| Hospitalization for unstable angina | 10 (0.3%) | 9 (0.3%) | |
The Kaplan-Meier based cumulative event probability is presented in Figure 1 for the time to first occurrence of the primary CV composite endpoint by treatment arm.
Figure 1: Kaplan-Meier Cumulative Curves of the Primary CV Endpoint (time to the first occurrence of the composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for unstable angina) – ITT Population
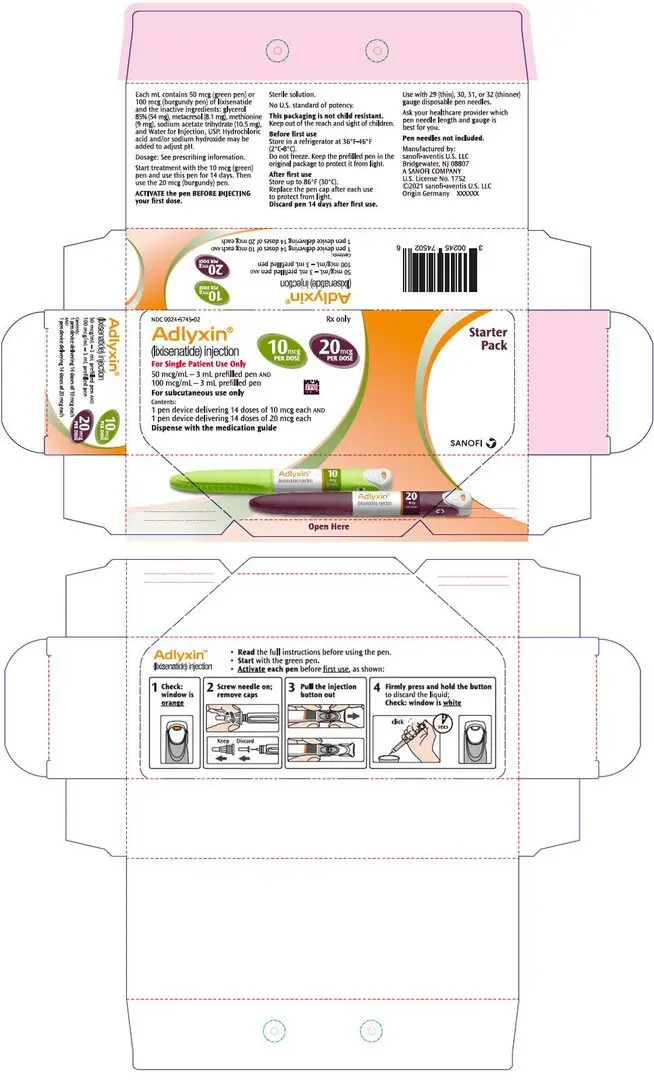
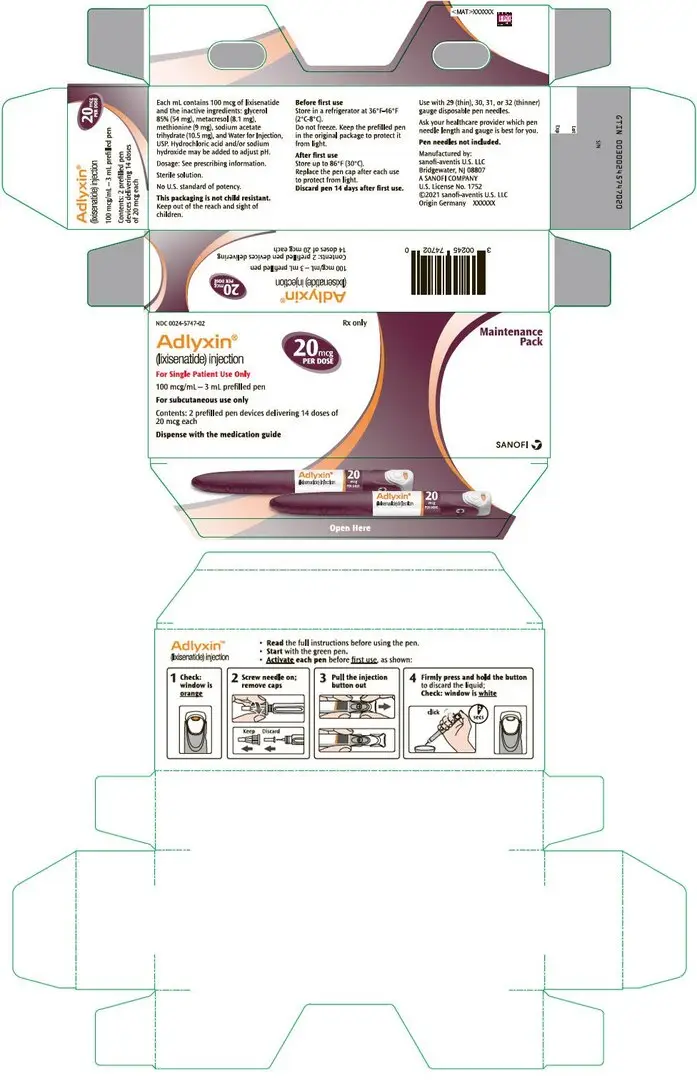
16. How is Adlyxin supplied
16.1 How Supplied
ADLYXIN (lixisenatide) injection is a clear and colorless solution supplied in a 3 mL single-patient-use pen.
The green starter pen is 50 mcg/mL and delivers 14 doses of 10 mcg. The burgundy maintenance pen is 100 mcg/mL and delivers 14 doses of 20 mcg.
The following packages are available:
- Starter Pack, NDC 0024-5745-02: For treatment initiation, Starter Pack contains 1 single-patient-use prefilled green pen of ADLYXIN 10 mcg (NDC 0024-5741-01) and 1 single-patient-use prefilled burgundy pen of ADLYXIN 20 mcg (NDC 0024-5740-00).
- Maintenance Pack, NDC 0024-5747-02: Contains 2 single-patient-use prefilled burgundy pens for ADLYXIN 20 mcg (NDC 0024-5740-00).
16.2 Storage and Handling
Prior to first use, ADLYXIN should be stored in a refrigerator, 36°F to 46°F (2°C to 8°C). Do not freeze. Keep the prefilled pen in the original package to protect it from light.
After first use, store up to 86°F (30°C). Replace the pen cap after each use to protect from light. Discard pen 14 days after first use.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
| MEDICATION GUIDE ADLYXIN® (ad-LIX-in) (lixisenatide) injection, for subcutaneous use |
|||
| What is the most important information I should know about ADLYXIN? | |||
| Do not share your ADLYXIN pen with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them. | |||
| ADLYXIN may cause serious side effects including inflammation of the pancreas (pancreatitis), which may be severe and lead to death. Before using ADLYXIN, tell your healthcare provider if you have had: |
|||
|
|
||
| These medical problems may make you more likely to get pancreatitis. Stop taking ADLYXIN and call your healthcare provider right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis. |
|||
| What is ADLYXIN? | |||
ADLYXIN is an injectable prescription medicine that may improve blood sugar (glucose) control in adults with type 2 diabetes, when used with diet and exercise.
|
|||
| Who should not use ADLYXIN? Do not use ADLYXIN if you:
|
|||
| Symptoms of a severe allergic reaction with ADLYXIN may include: | |||
|
|
||
Before using ADLYXIN, tell your healthcare provider about all of your medical conditions, including if you:
|
|||
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. | |||
Especially tell your healthcare provider if you take:
|
|||
| Ask your healthcare provider if you are not sure if your medicine is listed above. | |||
| Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine. | |||
How should I use ADLYXIN?
|
|||
| What are the possible side effects of ADLYXIN? | |||
ADLYXIN may cause serious side effects including:
|
|||
|
|
||
| Talk with your healthcare provider about how to treat low blood sugar. | |||
|
|||
|
|
||
| The most common side effects of ADLYXIN include: | |||
|
|
||
| These are not all the possible side effects of ADLYXIN. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
How should I store ADLYXIN?
Keep your ADLYXIN pen, pen needles, and all medicines out of the reach of children. |
|||
| General information about the safe and effective use of ADLYXIN. | |||
| Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ADLYXIN for a condition for which it was not prescribed. Do not give ADLYXIN to other people, even if they have the same symptoms that you have. It may harm them. | |||
| You can ask your pharmacist or healthcare provider for information about ADLYXIN that is written for health professionals. | |||
| What are the ingredients in ADLYXIN? | |||
| Active ingredient: lixisenatide | |||
| Inactive ingredients: glycerol 85%, metacresol, methionine, sodium acetate trihydrate, and Water for Injection, USP. Hydrochloric acid and sodium hydroxide solution are added as needed to adjust the pH. | |||
| Manufactured by: sanofi-aventis U.S. LLC, Bridgewater, NJ 08807, A SANOFI COMPANY | |||
| For more information, go to www.ADLYXIN.com or call sanofi-aventis at 1-800-633-1610. U.S. License No.1752 |
|||
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Revised: June 2022 | ||
INSTRUCTIONS FOR USE
ADLYXIN® (ad-LIX-in)
(lixisenatide)
Injection, for subcutaneous use
Starter pack - Contains 2 prefilled pens each with 14 doses.
One 10 microgram (mcg) pen, each dose contains 10 mcg in 0.2 mL.
One 20 microgram (mcg) pen, each dose contains 20 mcg in 0.2 mL.
Section 1 – Important Information
Read these instructions carefully before using your ADLYXIN pens.
Keep this leaflet for future reference.
Do not share your ADLYXIN pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
ADLYXIN pen Information
- ADLYXIN comes as a single-patient-use prefilled pen for injection.
- Inject 1 dose per day.
- There is no need to measure each dose.
- Talk with your healthcare provider about how to use the ADLYXIN pen and to inject correctly before using it.
- If you cannot follow all the instructions completely on your own or are not able to handle the pen (for example, if you have vision problems), only use it if you have help.
About Your ADLYXIN Starter Pack
The ADLYXIN Starter pack includes 2 different colored pens that contain different strengths of ADLYXIN. Both pens are used in the same way.
- The green pen contains 14 pre-set doses. Each dose contains 10 mcg of ADLYXIN.
- The burgundy pen contains 14 pre-set doses. Each dose contains 20 mcg of ADLYXIN.
You must start your treatment with the green 10 mcg ADLYXIN pen. You must first use all 14 doses from this pen. Then use the burgundy 20 mcg ADLYXIN pen.
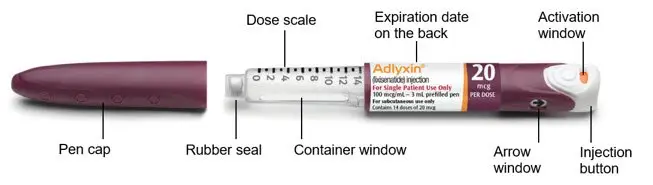
About Your ADLYXIN Pens
Green 10 mcg ADLYXIN pen
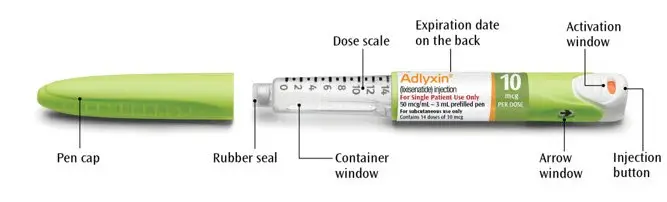

The plunger will move along the dose scale after each injection. In the example above, the dose number shows there are 13 injections left in the ADLYXIN pen.
Burgundy 20 mcg ADLYXIN pen
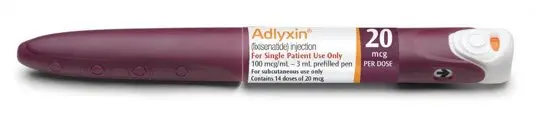
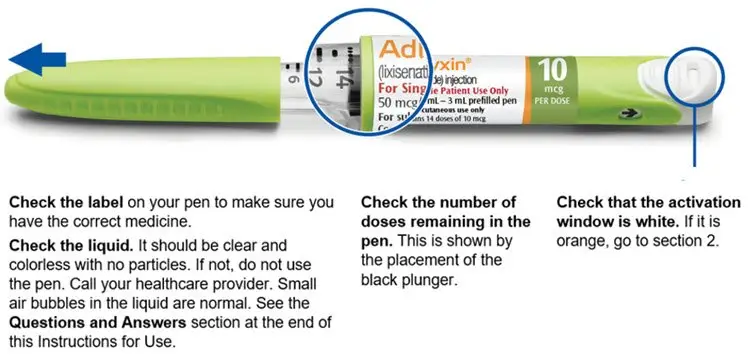
- Always check the label to make sure you have the correct ADLYXIN pen. Also, check that it has not passed the expiration date printed on the ADLYXIN pen. Do not use ADLYXIN past the expiration date. Using the wrong medicine could be harmful to your health.
- Inject ADLYXIN only by using this pen injector. Never use a syringe to withdraw ADLYXIN from the pen.
About your pen needles (supplied separately)
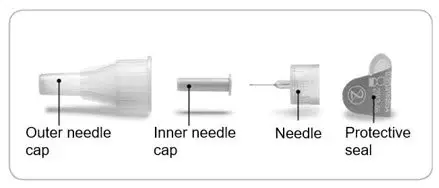
- Pen needles are not included with your pen.
- Always use a new needle for each injection. This helps prevent contamination of ADLYXIN or possible needle blockage.
- Only use needles that have been approved for use with ADLYXIN. The ADLYXIN pen may be used with certain pen needles from Becton Dickinson, Ypsomed, and Owen Mumford that are 8 mm long or shorter. Ask your healthcare provider which needle gauge and length is best for you.
- Do not reuse or share needles with another person.
Section 2 – Getting Started
- Begin with the green 10 mcg ADLYXIN pen.
- Do not activate the burgundy 20 mcg ADLYXIN pen until you have finished the green pen.
First activate your new pen
- Before injecting the first dose of ADLYXIN, you must activate the new pen. This is a one-time process called "activation."Step 1 to Step 5 below show you how to do this.
- Activation is done to make sure that the pen is working correctly and that the dose for your first injection is correct.
- Do not repeat the activation process or you will not receive 14 doses from your ADLYXIN pen.
The pictures below show how the injection button of your pen changes after activation.
| Before activation (orange window) | After activation (white window) |
|
|
|
| The pen is not activated when the activation window is orange. The pen must be activated before injecting your first dose of ADLYXIN. | The pen is activated and ready for injections when the activation window is white. |
How to activate your new ADLYXIN pen
Step 1. Pull off the pen cap and check the pen

Step 2. Screw needle on and remove needle caps

- Always use a new needle for activation.
- Remove the protective seal from the outer needle cap.
- Line up the needle with the pen. Push the outer needle cap containing the needle straight onto the pen, then screw the needle on until secure.
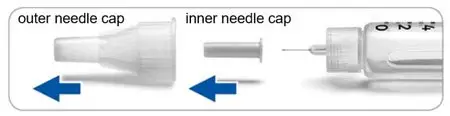
-
Pull off (do not unscrew) the outer needle cap.
- Pull off the inner needle cap and throw it away.
- Keep the outer needle cap to remove the needle later.
- Take care not to injure yourself when the needle is exposed.
Step 3. Pull the injection button out
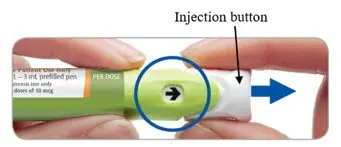
The arrow in the window will be pointing towards the injection button.
Pull the injection button out firmly until it stops.

The arrow will now be pointing towards the needle.
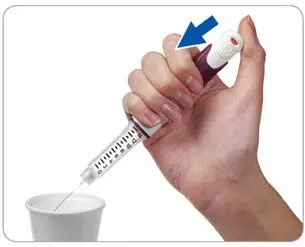
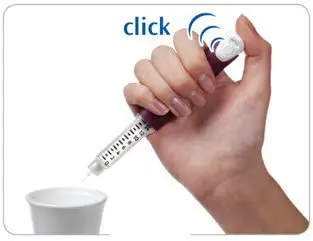
Step 4. Firmly press and hold the injection button to remove the liquid
|
|
|
|
|
|
- Do not inject into the body.
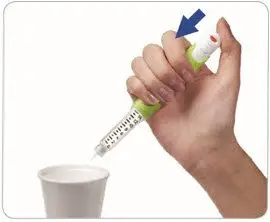
- Point the needle into a container (like a paper cup or tissue).
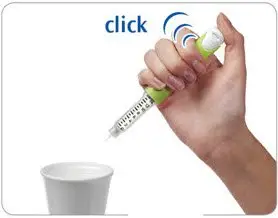
- Firmly press the injection button all the way in to discard the liquid. You may feel or hear a "click."
- Keep the injection button pressed in and slowly count to 2, which is equal to 2 seconds.
- If no liquid comes out of your pen, see the Questions and Answers section at the end of this Instructions for Use.
- Check that the activation window is now white.
Step 5. The pen is now ready for use.
- Do not activate this pen again.
For your first injection, go directly to Section 3 – Step 3.
- You do not need to replace the needle between activation and first injection if you inject yourself immediately after activation.
Section 3 – Daily use of pen
Inject only 1 dose each day.
Check to make sure the activation window is white before continuing in this section.

Step 1. Pull off pen cap and check pen
Step 2. Attach a new needle and remove the needle caps

- Always use a new needle for each injection.
- Remove the protective seal from the outer needle cap.
- Line up the needle with the pen. Put the outer needle cap containing the needle straight on the pen, then screw the needle on until secure.

-
Pull off (do not unscrew) the outer needle cap.
- Pull off the inner needle cap and throw it away.
- Keep the outer needle cap to remove the needle later.
- Take care not to injure yourself when the needle is exposed.
Step 3. Pull the injection button out
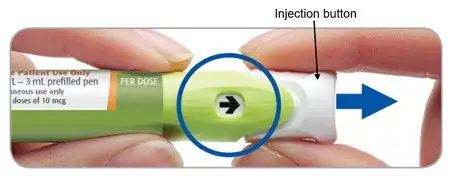
The arrow in the window will be pointing towards the injection button.
Pull the injection button out firmly until it stops.

The arrow will now be pointing towards the needle.
Step 4. Choosing Injection Sites
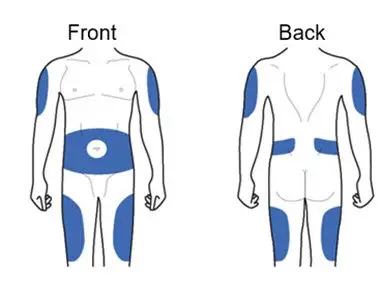
ADLYXIN must be injected under the skin and can be injected in any of the areas shown above in blue. These areas include the thigh, abdomen, or upper arm. Ask your healthcare provider about how to inject correctly.
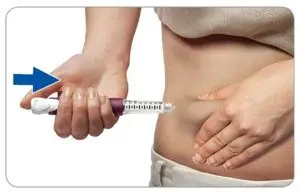
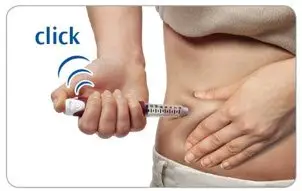
Step 5. Press and hold the injection button to inject the dose
|
|
|
|
|
|
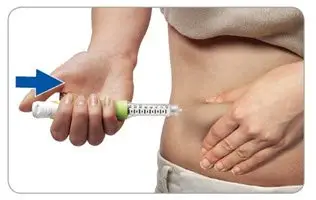
- Grasp a fold of skin and insert the needle (see the Injection sites section about where to inject).
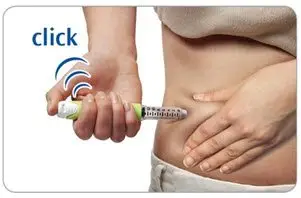
- Press the injection button all the way in. You may feel or hear a "click."
- Keep the injection button pressed in, hold the pen in place and slowly count to 2, which is equal to 2 seconds, before you pull the needle out of the skin.
If you do not hold the injection button in or if you remove the injector too early you may not get the full dose.
Your dose has now been given. Pull the needle out of your skin.
Step 6. Remove and throw away needle after each injection
- Grip the widest part of the outer needle cap. Keep the needle straight and guide it into the outer needle cap.
- Then push firmly on.
- The needle can puncture the cap if it is recapped at an angle.
- Grip and squeeze the widest part of the outer needle cap. Turn your pen several times with your other hand to remove the needle.
- Try again if the needle does not come off the first time.
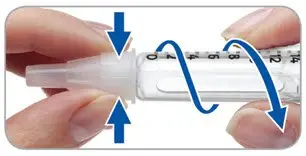
- Replace the pen cap.

- Put the needle in a puncture-resistant container (or as instructed by your healthcare provider).
Step 7. Repeat all steps in Section 3 for each injection.
Throw away a pen 14 days after activation, even if there is some medicine left.
After the green pen is thrown away continue to Section 4 to begin using the burgundy pen.
Section 4 – Moving to the burgundy pen
Completed use of the green 10 mcg pen
The green 10 mcg ADLYXIN pen is empty when the black plunger has reached "0" on the dose scale and the injection button cannot be pulled out fully.
After the green 10 mcg ADLYXIN pen is empty you must continue your treatment the next day using the burgundy 20 mcg ADLYXIN pen. This is used in exactly the same way.
Use of burgundy 20 mcg pen
Burgundy 20 mcg pen activation
The burgundy 20 mcg ADLYXIN pen must also be activated before use. Follow all steps in Section 2.
Burgundy 20 mcg pen use
To inject a dose with the burgundy 20 mcg ADLYXIN pen, follow all steps in Section 3. Repeat Section 3 for your daily injections until your pen is empty.
Table of activation and disposal
In the table write the date when you activated your pen and the date to throw it away 14 days later.
| Pen | Date of activation | Date to throw away |
|---|---|---|
| 10 mcg | ___ / ___ / ___ | ___ / ___ / ___ |
| 20 mcg | ___ / ___ / ___ | ___ / ___ / ___ |
Storage
General information
- Keep your ADLYXIN pens in a safe place out of the reach and sight of children.
- Protect your ADLYXIN pens from dust and dirt.
- Replace the pen cap after each use in order to protect the container window from light.
- Protect the ADLYXIN pen from light.
- Do not use ADLYXIN after the expiration date, which is stated on the label and on the carton. The expiration date refers to the last day of that month.
Before activation of the pen:
- Store your unused ADLYXIN pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze ADLYXIN pens and do not use ADLYXIN if it has been frozen.
- Allow your pen to warm at room temperature before using.
After activation of the pen:
- After activation, store your ADLYXIN pen at room temperature no higher than 86°F (30°C).
- Do not store your ADLYXIN pen with the needle attached. An attached needle might lead to contamination and may cause air bubbles which might affect your dose of medicine.
- After your ADLYXIN pen is activated it can be used for up to 14 days. Throw away a used ADLYXIN pen after 14 days. Do this even if there is some medicine left in the pen.
Throwing your pen away
- Replace the pen cap before throwing away (disposing of) your ADLYXIN pen.
- Put the used ADLYXIN pen in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the ADLYXIN pen and loose needles in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Maintenance
- Handle your ADLYXIN pen with care.
- You can clean the outside of your ADLYXIN pen by wiping it with a damp cloth.
- Do not soak, wash, or put liquid on (lubricate) your ADLYXIN pen. This may damage it.
- If you think your ADLYXIN pen may be damaged, do not use it. Get a new one. Do not try to repair the pen.
What do I do if I forget to activate the ADLYXIN pen or inject myself before activation?
If you have injected yourself before activating the pen, do not correct this by giving yourself a second injection. Contact your healthcare provider for advice on checking your blood sugar.
What do I do if there are air bubbles in the container?
Small air bubbles in the container are normal and they will not harm you. Your dose will be correct and you can keep following the instructions. Contact your healthcare provider if you need help.
What do I do if no liquid comes out during activation?
The needle may be blocked or not properly attached. Remove the needle from the pen, attach a new needle, and repeat Step 4 and Step 5 of Section 2 only. If still no liquid comes out, your ADLYXIN pen may be damaged. Do not use this ADLYXIN Starter pack. Contact your healthcare provider for help.
What do I do if it is hard to press the injection button all the way in?
The needle may be blocked or not properly attached. Pull the needle out of your skin and remove the needle from the pen. Attach a new needle and repeat Step 5 and Step 6 of Section 3 only. If it is still hard to press in the injection button, your ADLYXIN pen may be damaged. Do not use this ADLYXIN starter pack. Contact your healthcare provider for help.
Manufactured by:
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807
A SANOFI COMPANY
U.S. License No. 1752
©2021 sanofi-aventis U.S. LLC
If you have any questions about ADLYXIN or about diabetes, ask your healthcare provider or call sanofi-aventis U.S. LLC at 1-800-633-1610.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: July 2021
INSTRUCTIONS FOR USE
ADLYXIN® (ad-LIX-in)
(lixisenatide)
Injection, for subcutaneous use
One pre-filled pen contains 14 doses, each dose contains 20 micrograms (mcg) in 0.2 mL.
Section 1 – Important Information
Read these instructions carefully before using your ADLYXIN pen.
Keep this leaflet for future reference.
Do not share your ADLYXIN pen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
ADLYXIN pen Information
- ADLYXIN comes as a single-patient-use prefilled pen for injection.
- Inject 1 dose per day.
- There is no need to measure each dose.
- Talk with your healthcare provider about how to use the ADLYXIN pen and to inject correctly before using it.
- If you cannot follow all the instructions completely on your own or are not able to handle the pen (for example, if you have vision problems), only use it if you have help.
About Your ADLYXIN Pen
Burgundy 20 mcg ADLYXIN pen
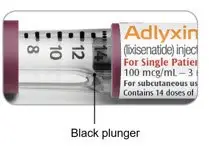
The plunger will move along the dose scale after each injection. In the example above, the dose number shows there are 13 injections left in the ADLYXIN pen.
- Always check the label to make sure you have the correct ADLYXIN pen. Also, check that it has not passed the expiration date printed on the ADLYXIN pen. Do not use ADLYXIN past the expiration date. Using the wrong medicine could be harmful to your health.
- Inject ADLYXIN only by using this pen injector. Never use a syringe to withdraw ADLYXIN from the pen.
About your pen needles (supplied separately)
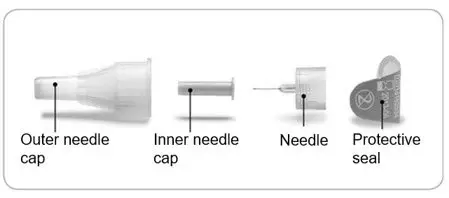
- Pen needles are not included with your pen.
- Always use a new needle for each injection. This helps prevent contamination of ADLYXIN or possible needle blockage.
- Only use needles that have been approved for use with ADLYXIN. The ADLYXIN pen may be used with certain pen needles from Becton Dickinson, Ypsomed, and Owen Mumford that are 8 mm long or shorter. Ask your healthcare provider which needle gauge and length is best for you.
- Do not reuse or share needles with another person.
Section 2 – Getting Started
- Activate the pen on the same day as your first injection with your new pen.
First activate your new pen
- Before injecting the first dose of ADLYXIN, you must activate the new pen. This is a one-time process called "activation."Step 1 to Step 5 below show you how to do this.
- Activation is done to make sure that the pen is working correctly and that the dose for your first injection is correct.
- Do not repeat the activation process or you will not receive 14 doses from your ADLYXIN pen.
The pictures below show how the injection button of your pen changes after activation.
| Before activation
(orange window) | After activation (white window) |
|
|
|
| The pen is not activated when the activation window is orange. The pen must be activated before injecting your first dose of ADLYXIN. | The pen is activated and ready for injections when the activation window is white. |
How to activate your new ADLYXIN pen
Step 1. Pull off the pen cap and check the pen

Step 2. Screw needle on and remove needle caps

- Always use a new needle for activation.
- Remove the protective seal from the outer needle cap.
- Line up the needle with the pen. Push the outer needle cap containing the needle straight onto the pen, then screw the needle on until secure.

-
Pull off (do not unscrew) the outer needle cap.
- Pull off the inner needle cap and throw it away.
- Keep the outer needle cap to remove the needle later.
- Take care not to injure yourself when the needle is exposed.
Step 3. Pull the injection button out
The arrow in the window will be pointing towards the injection button.
Pull the injection button out firmly until it stops.

The arrow will now be pointing towards the needle.
Step 4. Firmly press and hold the injection button to remove the liquid
|
|
|
|
|
|
- Do not inject into the body.
- Point the needle into a container (like a paper cup or tissue).
- Firmly press the injection button all the way in to discard the liquid. You may feel or hear a "click."
- Keep the injection button pressed in and slowly count to 2, which is equal to 2 seconds.
- If no liquid comes out of your pen, see the Questions and Answers section at the end of this Instructions for Use.
- Check that the activation window is now white.
Step 5. The pen is now ready for use.
- Do not activate this pen again.
For your first injection, go directly to Section 3 – Step 3.
- You do not need to replace the needle between activation and first injection if you inject yourself immediately after activation.
Section 3 – Daily use of pen
Inject only 1 dose each day.
Check to make sure the activation window is white before continuing in this section.

Step 1. Pull off pen cap and check pen

Step 2. Attach a new needle and remove the needle caps

- Always use a new needle for each injection.
- Remove the protective seal from the outer needle cap.
- Line up the needle with the pen. Put the outer needle cap containing the needle straight on the pen, then screw the needle on until secure.

-
Pull off (do not unscrew) the outer needle cap.
- Pull off the inner needle cap and throw it away.
- Keep the outer needle cap to remove the needle later.
- Take care not to injure yourself when the needle is exposed.
Step 3. Pull the injection button out
The arrow in the window will be pointing towards the injection button.
Pull the injection button out firmly until it stops.

The arrow will now be pointing towards the needle.
Step 4. Choosing Injection Sites
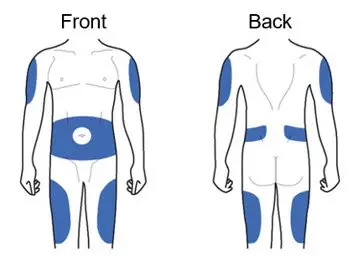
ADLYXIN must be injected under the skin and can be injected in any of the areas shown above in blue. These areas include the thigh, abdomen, or upper arm. Ask your healthcare provider about how to inject correctly.
Step 5. Press and hold the injection button to inject the dose
|
|
|
|
|
|
- Grasp a fold of skin and insert the needle (see the Injection sites section about where to inject).
- Press the injection button all the way in. You may feel or hear a "click."
- Keep the injection button pressed in, hold the pen in place and slowly count to 2, which is equal to 2 seconds, before you pull the needle out of the skin.
If you do not hold the injection button in or if you remove the injector too early you may not get the full dose.
Your dose has now been given. Pull the needle out of your skin.
Step 6. Remove and throw away needle after each injection
- Grip the widest part of the outer needle cap. Keep the needle straight and guide it into the outer needle cap.
- Then push firmly on.
- The needle can puncture the cap if it is recapped at an angle.
- Grip and squeeze the widest part of the outer needle cap. Turn your pen several times with your other hand to remove the needle.
- Try again if the needle does not come off the first time.
- Replace the pen cap.

- Put the needle in a puncture-resistant container (or as instructed by your healthcare provider).
Step 7. Repeat all steps in Section 3 for each injection.
Throw away a pen 14 days after activation, even if there is some medicine left.
Table of activation and disposal
In the table, write the date when you activated your pen and the date to throw it away 14 days later.
| Pen | Date of activation | Date to throw away |
|---|---|---|
| 1 | ||
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 |
Storage
General information
- Keep your ADLYXIN pen in a safe place out of the reach and sight of children.
- Protect your ADLYXIN pen from dust and dirt.
- Replace the pen cap after each use in order to protect the container window from light.
- Protect the ADLYXIN pen from light.
- Do not use ADLYXIN after the expiration date, which is stated on the label and on the carton. The expiration date refers to the last day of that month.
Before activation of the pen:
- Store your unused ADLYXIN pens in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze ADLYXIN pens and do not use ADLYXIN if it has been frozen.
- Allow your pen to warm at room temperature before using.
After activation of the pen:
- After activation, store your ADLYXIN pen at room temperature no higher than 86°F (30°C).
- Do not store your ADLYXIN pen with the needle attached. An attached needle might lead to contamination and may cause air bubbles which might affect your dose of medicine.
- After your ADLYXIN pen is activated it can be used for up to 14 days. Throw away a used ADLYXIN pen after 14 days. Do this even if there is some medicine left in the pen.
Throwing your pen away
- Replace the pen cap before throwing away (disposing of) your ADLYXIN pen.
- Put the used ADLYXIN pen in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the ADLYXIN pen and loose needles in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Maintenance
- Handle your ADLYXIN pen with care.
- You can clean the outside of your ADLYXIN pen by wiping it with a damp cloth.
- Do not soak, wash, or put liquid on (lubricate) your ADLYXIN pen. This may damage it.
- If you think your ADLYXIN pen may be damaged, do not use it. Get a new one. Do not try to repair the pen.
What do I do if I forget to activate the ADLYXIN pen or inject myself before activation?
If you have injected yourself before activating the pen, do not correct this by giving yourself a second injection. Contact your healthcare provider for advice on checking your blood sugar.
What do I do if there are air bubbles in the container?
Small air bubbles in the container are normal and they will not harm you. Your dose will be correct and you can keep following the instructions. Contact your healthcare provider if you need help.
What do I do if no liquid comes out during activation?
The needle may be blocked or not properly attached. Remove the needle from the pen, attach a new needle, and repeat Step 4 and Step 5 of Section 2 only. If still no liquid comes out, your ADLYXIN pen may be damaged. Do not use this ADLYXIN pen. Contact your healthcare provider for help.
What do I do if it is hard to press the injection button all the way in?
The needle may be blocked or not properly attached. Pull the needle out of your skin and remove the needle from the pen. Attach a new needle and repeat Step 5 and Step 6 of Section 3 only. If it is still hard to press in the injection button, your ADLYXIN pen may be damaged. Do not use this ADLYXIN pen. Contact your healthcare provider for help.
Manufactured by:
sanofi-aventis U.S. LLC
Bridgewater, NJ 08807 A SANOFI COMPANY
U.S. License No. 1752
©2021 sanofi-aventis U.S. LLC
If you have any questions about ADLYXIN or about diabetes, ask your healthcare provider or call sanofi-aventis U.S. LLC at 1-800-633-1610.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: July 2021
| ADLYXIN
lixisenatide kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ADLYXIN
lixisenatide injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (824676584) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sanofi-Aventis Deutschland GmbH | 313218430 | ANALYSIS(0024-5745, 0024-5747) , MANUFACTURE(0024-5745, 0024-5747) , API MANUFACTURE(0024-5745, 0024-5747) , PACK(0024-5745, 0024-5747) , LABEL(0024-5745, 0024-5747) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | LABEL(0024-5745, 0024-5747) , PACK(0024-5745, 0024-5747) | |