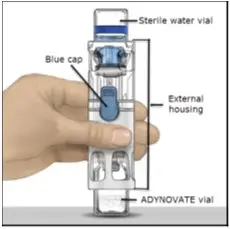
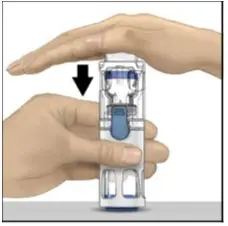
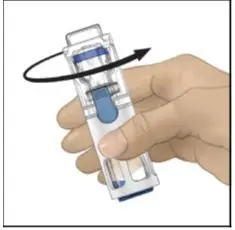
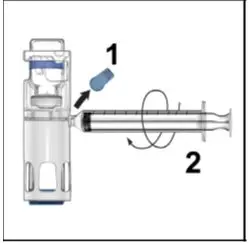
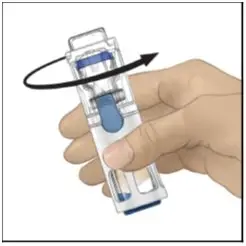
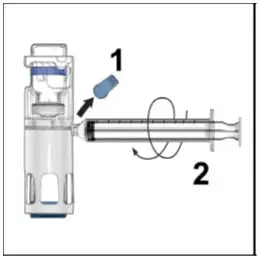
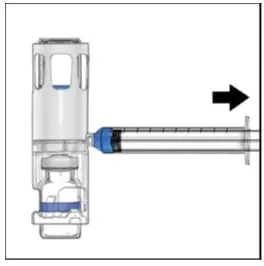
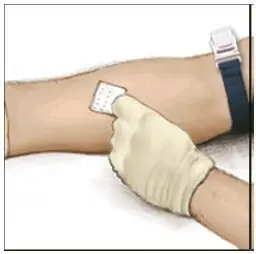
Drug Detail:Adynovate (recombinant) (Antihemophilic factor (recombinant) [ ant-ee-hee-moe-fil-ik-fak-tor ])
Drug Class: Miscellaneous coagulation modifiers
Highlights of Prescribing Information
ADYNOVATE, (Antihemophilic Factor, Recombinant, PEGylated) Lyophilized Powder for Solution For Intravenous Injection
Initial U.S. Approval: 2015
Recent Major Changes
| Warnings and Precautions (5.1) | 8/2023 |
Indications and Usage for Adynovate
ADYNOVATE, Antihemophilic Factor (Recombinant), PEGylated, is a human antihemophilic factor indicated in children and adults with hemophilia A (congenital factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes
- Perioperative management
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitation of Use
ADYNOVATE is not indicated for the treatment of von Willebrand disease. (1)
Adynovate Dosage and Administration
For intravenous use after reconstitution only.
- One unit per kilogram body weight will raise the factor VIII level by 2% international units per deciliter (IU per dL). Each vial of ADYNOVATE is labeled with the actual amount of recombinant factor VIII present in IU. (2.1)
- On-demand treatment and control of bleeding episodes and perioperative management:
- Estimated Increment of factor VIII (IU/dL or % of normal) = [Total Dose (IU)/body weight (kg)] × 2 (IU/dL per IU/kg)
- Dose (IU) = Body Weight (kg) × Desired factor VIII Rise (IU/dL or % of Normal) × 0.5 (IU/kg per IU/dL)
- Administer 40-50 IU/kg body weight twice weekly in adults and adolescents (12 years and older).
- Administer 55 IU/kg twice weekly in children (<12 years) with a maximum of 70 IU/kg
- Adjust the dose and dosing intervals based on the patient's clinical response.
- Inject intravenously over a period of less than or equal to 5 minutes (maximum infusion rate 10 mL per min). (2.3)
Dosage Forms and Strengths
ADYNOVATE is available as a lyophilized powder in single-dose vials containing nominally (approximately) 250, 500, 750, 1000, 1500, 2000, or 3000 international units. (3)
Contraindications
Do not use in patients who have had prior anaphylactic reaction to ADYNOVATE, the parent molecule (ADVATE), mouse or hamster protein, or excipients of ADYNOVATE. (4)
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, have been reported. Should symptoms occur, discontinue treatment with ADYNOVATE and administer appropriate treatment. (5.1)
- Development of factor VIII neutralizing antibodies (inhibitors) may occur. If expected plasma factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor VIII inhibitor concentration. (5.2, 5.3)
Adverse Reactions/Side Effects
The most common adverse reactions reported in ≥1% of subjects in the clinical studies were headache, diarrhea, rash, nausea dizziness and urticaria. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pediatric Use: Higher clearance, a shorter half-life and lower incremental recovery of factor VIII has been observed in children (<12 years). Dose adjustment or more frequent dosing based on per kg body weight may be needed in this population. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
Full Prescribing Information
1. Indications and Usage for Adynovate
ADYNOVATE, Antihemophilic Factor (Recombinant), PEGylated, is a human antihemophilic factor indicated in children and adults with hemophilia A (congenital factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes
- Perioperative management
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitation of Use
ADYNOVATE is not indicated for the treatment of von Willebrand disease.
2. Adynovate Dosage and Administration
For intravenous use after reconstitution only.
2.1 Dose
- Each vial label of ADYNOVATE states the actual factor VIII potency in international units. This may be more or less than the nominal vial potency/content. One international unit corresponds to the activity of factor VIII contained in one milliliter of normal human plasma.
- Dosage and duration of treatment depend on the severity of factor VIII deficiency, the location and extent of the bleeding, and the patient's clinical condition. Careful monitoring of replacement therapy is necessary in cases of serious or life-threatening bleeding episodes.
- Potency assignment is determined using a one-stage clotting assay. Plasma factor VIII levels can be monitored clinically using a one-stage clotting assay.
- Calculate the dose of ADYNOVATE based on the empirical finding that one international unit of ADYNOVATE per kg body weight increases the plasma factor VIII level by 2 IU per dL of plasma. Use the following formula to estimate the expected in vivo peak increase in factor VIII level expressed as IU per dL (or % of normal) and the dose to achieve a desired in vivo peak increase in factor VIII level:
Estimated Increment of factor VIII (IU/dL or % of normal) = [Total Dose (IU)/body weight (kg)] × 2 (IU/dL per IU/kg)
Dose (IU) = Body Weight (kg) × Desired factor VIII Rise (IU/dL or % of Normal) × 0.5 (IU/kg per IU/dL)
- Patients vary in their pharmacokinetic (e.g., clearance, half-life, in vivo recovery) and clinical response. Base the dose and frequency of ADYNOVATE on the individual clinical response.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing of ADYNOVATE for the on-demand treatment and control of bleeding episodes is provided in Table 1. Maintain plasma factor VIII activity level at or above the described plasma levels (in IU per dL or % of normal).
| Type of Bleeding | Target Factor VIII Level (IU/dL or % of normal) | Dose*
(IU/kg) | Frequency of Dosing (hours) | Duration of Therapy |
|---|---|---|---|---|
|
||||
| Minor Early hemarthrosis, mild muscle bleeding, or mild oral bleeding episode. | 20-40 | 10-20 | 12-24 | Until the bleeding is resolved |
| Moderate Muscle bleeding, moderate bleeding into the oral cavity, definite hemarthroses, and known trauma. | 30-60 | 15-30 | 12-24 | Until the bleeding is resolved |
| Major Significant gastrointestinal bleeding, intracranial, intra-abdominal or intrathoracic bleeding, central nervous system bleeding, bleeding in the retropharyngeal or retroperitoneal spaces or iliopsoas sheath, fractures, head trauma. | 60-100 | 30-50 | 8-24 | Until bleeding is resolved. |
Perioperative Management
A guide for dosing ADYNOVATE during surgery (perioperative management) is provided in Table 2. Consideration should be given to maintain a factor VIII activity at or above the target range.
| Type of Surgery | Factor VIII Level Required (% of normal or IU/dL) | Dose (IU/kg) | Frequency of Doses (hours) | Duration of Treatment |
|---|---|---|---|---|
| Minor
Including tooth extraction | 60-100 | 30-50 | Within one hour before surgery. Repeat after 24 hours if necessary | Single dose or repeat as needed until bleeding is resolved. |
| Major
Intracranial, intra-abdominal, or intrathoracic surgery, joint replacement surgery | 80-120 (pre- and post-operative) | 40-60 | Within one hour before the operation to achieve 100% activity. Repeat every 8 to 24 hours (6 to 24 hours for patients <12 years of age) to maintain FVIII activity within the target range | Until adequate wound healing |
2.3 Administration
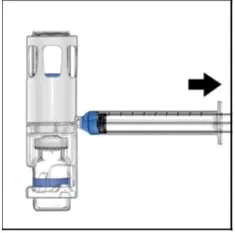
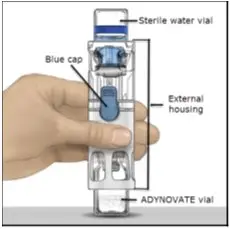
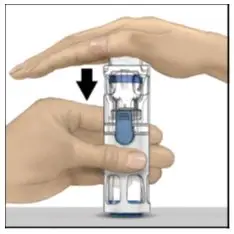
- Visually inspect the reconstituted ADYNOVATE solution for particulate matter and discoloration prior to administration, whenever solution and container permit. The final ADYNOVATE solution should be clear and colorless. Do not use if particulate matter or discoloration is observed.
- Administer ADYNOVATE as soon as possible, but no later than 3 hours after reconstitution.
3. Dosage Forms and Strengths
ADYNOVATE is a lyophilized powder in single-dose vials containing nominally (approximately) 250, 500, 750, 1000, 1500, 2000, and 3000 International Units (IU, units). The 250-1500 IU strengths come with 2 mL Sterile Water for Injection (sWFI); the 2000 and 3000 IU strengths come with 5 mL of sWFI. The actual factor VIII potency/content is labeled on each ADYNOVATE vial.
The potency assignment employs a factor VIII concentrate standard that is referenced to a WHO (World Health Organization) international standard for factor VIII concentrates and is evaluated by appropriate methodology to ensure accuracy of the results.
4. Contraindications
ADYNOVATE is contraindicated in patients who have had prior anaphylactic reaction to ADYNOVATE, to the parent molecule (ADVATE), mouse or hamster protein, or excipients of ADYNOVATE (e.g. Tris, mannitol, trehalose, glutathione, and/or polysorbate 80).
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Hypersensitivity reactions including anaphylaxis, have been reported with ADYNOVATE. Hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest tightness, dyspnea, wheezing, urticaria, pruritus, and nausea and vomiting. Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur.
5.2 Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to factor VIII can occur following administration of ADYNOVATE. Monitor patients regularly for the development of factor VIII inhibitors by appropriate clinical observations and laboratory tests. Perform an assay that measures factor VIII inhibitor concentration if the plasma factor VIII level fails to increase as expected, or if bleeding is not controlled with expected dose.
5.3 Monitoring Laboratory Tests
- Monitor plasma factor VIII activity by performing a validated one-stage clotting assay to confirm the adequate factor VIII levels have been achieved and maintained [see Dosage and Administration (2)].
- Monitor for the development of factor VIII inhibitors. Perform the Bethesda inhibitor assay to determine if factor VIII inhibitor is present. If expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with the expected dose of ADYNOVATE, use Bethesda Units (BU) to determine inhibitor levels.
6. Adverse Reactions/Side Effects
The most common adverse reactions (≥1% of subjects) reported in the clinical studies were headache, diarrhea, rash, nausea, dizziness and urticaria.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ADYNOVATE was evaluated in 365 previously treated patients (PTPs) and previously untreated patients (PUPs) with severe hemophilia A (factor VIII less than 1% of normal), who received at least one dose of ADYNOVATE in 6 completed multi-center, prospective, open label clinical studies and 1 ongoing clinical studies. The total number of infusions within the safety database is 74487. Table 3 lists the adverse reactions reported during clinical studies.
| MedDRA System Organ Class | MedDRA Preferred Term | Number of Subjects n (%) (N=365) |
|---|---|---|
|
||
| Gastrointestinal Disorders | Diarrhea | 25 (6.8%) |
| Nausea | 8 (2.2%) | |
| Eye Disorders | Ocular Hyperaemia | 3 (0.8%) |
| Immune System Disorder | Hypersensitivity* | 2 (0.5%) |
| Nervous System Disorders | Headache | 41 (11.2%) |
| Dizziness | 7 (1.9%) | |
| Skin and Subcutaneous Tissue Disorders | Rash | 10 (2.7%) |
| Urticaria | 7 (1.9%) | |
| Rash Pruritic | 1 (0.3%) | |
| Vascular Disorders | Flushing | 1 (0.27%) |
| Investigations | Eosinophil Count Increased | 2 (0.5%) |
| Injury, Poisoning and Procedural Complications | Infusion Related Reaction | 2 (0.5%) |
Two cases of acute pancreatitis, with no precipitating cause identified in one case, were reported in adults during an extension study of the clinical trial which evaluated 216 subjects. Administration of ADYNOVATE continued and both cases resolved.
6.2 Immunogenicity
Clinical trial subjects were monitored for neutralizing (inhibitory) antibodies to FVIII. Of the 6 completed clinical trials in previously treated patients (PTPs), in the randomized controlled trial comparing different dosing regimens of Adynovate, one previously treated patient developed a transient low titer FVIII inhibitor at 0.6 BU while receiving more frequent dosing with Adynovate. In a continuation study with Adynovate, one patient developed a transient low titer (0.6BU) FVIII inhibitor. Repeat testing did not confirm the presence of inhibitor. Both of these subjects continued treatment without change in the dose of Adynovate.
Immunogenicity also was evaluated by measuring the development of binding IgG and IgM antibodies against factor VIII, PEGylated (PEG)-factor VIII, PEG and Chinese hamster ovary (CHO) protein using validated ELISA assays. Persistent treatment-emergent binding antibodies against FVIII, PEG-FVIII or PEG were not detected. Out of 365 subjects, thirty six subjects in total showed pre-existing antibodies to factor VIII (n=5), PEG-factor VIII (n=31) and/or PEG (n=6) prior to the first exposure to ADYNOVATE. Twenty four subjects who tested negative at screening developed transient antibodies against factor VIII (n= 10), PEG-FVIII (n= 16) and/or PEG (n=3) at one or two consecutive study visits. Antibodies were transient and not detectable at subsequent visits. Two subjects showed positive results for binding antibodies at study completion or at the time of data cutoff. Binding antibodies that were detected prior to exposure to ADYNOVATE, that transiently developed during the trial or were still detectable at study completion or data cutoff could not be correlated to any impaired treatment efficacy or altered PK parameters. There was no causal relationship between observed adverse events and binding antibodies except in one subject where a causal relationship cannot be ruled out based on available data. No subject had pre-existing or treatment-emergent antibodies to CHO protein.
From an ongoing study in previously untreated patients <6 years with severe hemophilia A, 9 cases of FVIII inhibitor development associated with treatment with Adynovate were reported.
The detection of antibodies that are reactive to factor VIII is highly dependent on many factors, including: the sensitivity and specificity of the assay, sample handling, timing of sample collection, concomitant medications and underlying disease. For these reasons, comparison of the incidence of antibodies to ADYNOVATE with the incidence of antibodies to other products may be misleading.
6.3 Postmarketing Experience
Because post-marketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate the frequency of these reactions or establish a causal relationship to product exposure.
Immune System Disorders: Anaphylactic Reaction
8. Use In Specific Populations
8.4 Pediatric Use
Safety and efficacy studies have been performed in 91 previously treated, pediatric patients age 1 year to <18 years who received at least one dose of ADYNOVATE as part of routine prophylaxis, on-demand treatment of bleeding episodes, or perioperative management. Adolescent subjects age 12 to <18 (n=25) were enrolled in the adult and adolescent safety and efficacy trial, and subjects <12 years of age (n=66) were enrolled in a pediatric trial. The safety and efficacy of ADYNOVATE in routine prophylaxis and the treatment of bleeding episodes were comparable between children and adults. [see Clinical Studies (14)]
Pharmacokinetic studies in children (<12 years) have demonstrated higher clearance, a shorter half-life and lower incremental recovery of factor VIII compared to adults. Because clearance (based on per kg body weight) has been demonstrated to be higher in children (<12 years), dose adjustment or more frequent dosing based on per kg body weight may be needed in this population. [see Clinical Pharmacology (12.3)]
11. Adynovate Description
ADYNOVATE, Antihemophilic Factor (Recombinant), PEGylated, is formulated as a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution for intravenous injection. The product is supplied in single-dose vials containing nominal (approximate) potencies of 250, 500, 750, 1000, 1500, 2000, or 3000 international units (IU). Each vial of ADYNOVATE is labeled with the actual factor VIII activity in IU determined using one-stage clotting assay, using a reference material calibrated against a World Health Organization (WHO) International Standard for factor VIII concentrates. One IU, as defined by the WHO standard for blood coagulation factor VIII, human, is approximately equal to the level of factor VIII activity found in 1 mL of fresh pooled human plasma.
When reconstituted with 2 mL or 5 mL sterile water for injection, the final solution contains the following excipients and stabilizers in targeted amounts per mL of reconstituted product:
| Stabilizer and Excipient | 2 mL Reconstitution (for 250, 500, 750, 1000, 1500 IU) Target (per mL) | 5 mL Reconstitution (for 2000, 3000 IU) Target (per mL) |
|---|---|---|
| Tris (hydroxymethyl) aminomethane | 3.05 mg | 1.22 mg |
| Calcium Chloride | 0.60 mg | 0.24 mg |
| Mannitol | 80 mg | 32 mg |
| Sodium Chloride | 13.15 mg | 5.26 mg |
| Trehalose Dihydrate | 20 mg | 8 mg |
| Glutathione | 0.2 mg | 0.08 mg |
| Histidine | 3.90 mg | 1.56 mg |
| Polysorbate 80 | 0.25 mg | 0.10 mg |
ADYNOVATE contains no preservative. The specific activity of ADYNOVATE is 2700 - 8000 IU/mg protein.
ADYNOVATE is a recombinant full-length human coagulation factor VIII (2,332 amino acids with a molecular weight (MW) of 280 kDa) covalently conjugated with one or more molecules of polyethylene glycol (MW 20 kDa) [see Clinical Pharmacology (12.1)]. The therapeutic activity of ADYNOVATE is derived from its parent drug substance, ADVATE [Antihemophilic Factor (Recombinant)], which is produced by recombinant DNA technology from the CHO cell line. ADVATE is purified from the culture medium using a series of chromatography columns. The purification process includes an immunoaffinity chromatography step in which a monoclonal antibody directed against factor VIII is employed to selectively isolate the factor VIII from the medium. The production process includes a dedicated, viral inactivation solvent-detergent treatment step. The ADVATE molecule is then covalently conjugated with the polyethylene glycol, which mainly targets lysine residues.
The cell culture, pegylation, purification process and formulation used in the manufacture of ADYNOVATE do not use additives of human or animal origins.
12. Adynovate - Clinical Pharmacology
12.1 Mechanism of Action
ADYNOVATE, a PEGylated form of recombinant antihemophilic factor (ADVATE), [see Description (11)], temporarily replaces the missing coagulation factor VIII needed for effective hemostasis in congenital hemophilia A patients. ADYNOVATE exhibits an extended terminal half-life through pegylation of the parent molecule, ADVATE, which reduces binding to the physiological factor VIII clearance receptor (LRP1).
12.2 Pharmacodynamics
Hemophilia A is a disorder characterized by a deficiency of functional coagulation factor VIII, resulting in a prolonged, patient plasma clotting time as measured by the activated partial thromboplastin time (aPTT). Treatment with ADYNOVATE normalizes the aPTT over the effective dosing period. The administration of ADYNOVATE increases plasma levels of factor VIII and can temporarily correct the coagulation defect in hemophilia A patients.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of ADYNOVATE were evaluated in a multi-center, prospective, open label clinical trial and compared with ADVATE in 26 subjects prior to initiation of prophylactic treatment with ADYNOVATE and in 22 subjects after 6 months of treatment with ADYNOVATE. A single dose of 45 IU/kg was utilized for both products. The PK parameters, as shown in Table 4, were based on plasma coagulation factor VIII activity measured by the one-stage clotting assay and are presented by age groups.
Incremental recovery was comparable between both products. The PK parameters determined after 6 months of prophylactic treatment with ADYNOVATE were consistent with the initial parameter estimates.
Pediatric Pharmacokinetics
Pharmacokinetic parameters calculated from 39 subjects <18 years of age (intent-to-treat analysis) are available for 14 children (2 to <6 years), 17 older children (6 to <12 years) and 8 adolescent subjects (12 to <18 years of age), as shown in Table 4. The mean clearance (based on body weight) of ADYNOVATE was higher and the mean half-life was lower in children <12 years of age than adults. A dose adjustment may be required in children <12 years of age.
| PK Parameters | Pediatric Population PK with Sparse Sampling* | Adult and Adolescent Individual PK with Full Sampling† |
||
|---|---|---|---|---|
| <6 years N=14 | 6 to <12 years N=17 | 12 to <18 years N = 8 | ≥18 years N = 18 |
|
| Abbreviations: MRT: mean residence time; CL: clearance; CI: confidence interval; AUC: area under the curve; Vss: body weight adjusted volume of distribution at steady-state; Cmax: maximum observed activity; Tmax: time to reach the maximum concentration. | ||||
|
||||
| Terminal half-life [h] | 11.8 ± 2.43 | 12.4 ± 1.67 | 13.43 ± 4.05 | 14.69 ± 3.79 |
| MRT [h] | 17.0 ± 3.50 | 17.8 ± 2.42 | 17.96 ± 5.49 | 20.27 ± 5.23 |
| CL [mL/(kg∙h)] | 3.53 ± 1.29 | 3.11 ± 0.76 | 3.87 ± 3.31 (2.73 ± 0.93)‡ | 2.27 ± 0.84 |
| Incremental Recovery [(IU/dL)/(IU/kg)] | 1.89 ± 0.49 | 1.95 ± 0.47 | 2.12 ± 0.60 | 2.66 ± 0.68 |
| AUC0-Inf [IU∙h/dL] | 1947 ± 757 | 2012 ± 495 | 1642 ± 752 | 2264 ± 729 |
| Vss [dL/kg] | 0.56 ± 0.12 | 0.54 ± 0.09 | 0.56 ± 0.18 | 0.43 ± 0.11 |
| Cmax [IU/dL] | 115 ± 30 | 115 ± 33 | 95 ± 25 | 122 ± 29 |
| Tmax [h] | -§ | -§ | 0.26 ± 0.10 | 0.46 ± 0.29 |
14. Clinical Studies
On-demand Treatment and Control of Bleeding Episodes
A total of 518 bleeding episodes were treated with ADYNOVATE in the per-protocol population, i.e. dosed according to the protocol specific dosing requirements. Of these, 361 bleeding episodes (n=17 subjects) occurred in the on-demand arm and 157 (n=61 subjects) occurred in the prophylaxis arm. The median dose per infusion to treat all bleeding episodes in the per-protocol population was 29 (Q1: 20.0; Q3: 39.2) IU per kg. The median dose per infusion to treat a minor, moderate, or severe/major bleeding episode in the per-protocol population was 25.5 (Q1: 16.9; Q3: 37.6) IU/kg, 30.9 (Q1: 23.0; Q3: 43.1) IU/kg, or 36.4 (Q1: 29.0; Q3: 44.5) IU/kg, respectively.
A total of 591 bleeding episodes were treated with ADYNOVATE in the treated population, which was identical to the safety analysis set of subjects assigned to routine prophylaxis or on-demand treatment with ADYNOVATE and who received at least one dose of the product. Of these, 361 bleeding episodes (n=17 subjects) occurred in the on-demand arm and 230 bleeding episodes (n=75 subjects) occurred in the routine prophylaxis arm. Efficacy in control of bleeding episodes is summarized in Table 5.
| Bleeding Episode Etiology | All | Joint | Non-joint | |
|---|---|---|---|---|
|
||||
| Number of bleeds treated | 591 | 455 | 136 | |
| Number of infusions to treat bleeding episodes | 1 infusions: | 85.4% | 85.9% | 83.8% |
| 2 infusions: | 10.8% | 10.8% | 11.0% | |
| Total (1 or 2 infusions): | 96.2% | 96.7% | 94.8% | |
| Rate of success to treat bleeding episodes* | Excellent or good | 95.3% | 95.8% | 93.4% |
Routine Prophylaxis
A total of 120 subjects (treated population) received a twice a week regimen in the prophylaxis arm, and an additional 17 subjects were treated episodically in the on-demand arm. In the treated population, the median [mean] annualized bleed rate (ABR) in the on-demand treatment arm was 41.5 [40.8] compared to 1.9 [4.7] while on a twice a week prophylaxis regimen (Table 6). In the per-protocol population, the median [mean] annualized bleed rate (ABR) in the on-demand treatment arm was 41.5 [40.8] compared to 1.9 [3.7] while on a twice a week prophylaxis regimen. Using a negative binomial model to estimate the ABR, there was a significant reduction in the ABR (p <0.0001) for subjects in the prophylaxis arm compared to the on-demand arm.
| Bleeding Episode Etiology | On-Demand Treatment | Routine Prophylaxis Treatment | ||
|---|---|---|---|---|
| Median | Mean (SD) | Median | Mean (SD) | |
| Overall | 41.5 | 40.8 (16.3) | 1.9 | 4.7 (8.6) |
| Joint | 38.1 | 34.7 (15.1) | 0.0 | 2.9 (8.0) |
| Non-Joint | 3.7 | 6.1 (6.7) | 0.0 | 1.8 (3.0) |
| Spontaneous | 21.6 | 26.0 (19.6) | 0.0 | 2.9 (7.1) |
| Traumatic | 9.3 | 14.9 (15.3) | 0.0 | 1.8 (3.1) |
In the treated population, the median [mean] ABR for the 23 adolescent subjects age 12 to <18 years of age on routine prophylaxis was 2.1 [5.2] compared to a median [mean] ABR of 1.9 [4.6] for the 97 subjects 18 years and older. Reduction in ABR between the treatment arms was observed regardless of baseline subgroups examined, including age, presence or absence of target joints, and pre-trial treatment regimen. The majority of the bleeding episodes during prophylaxis (95%) were of minor/moderate severity. Forty-five out of 120 subjects (38%) experienced no bleeding episodes and 68 out of 120 subjects (57%) experienced no joint bleeding episodes in the prophylaxis arm. Of those subjects who were compliant to regimen (per-protocol population), 40 out of 101 subjects (40%) experienced no bleeding episodes. All subjects in the on-demand arm experienced a bleeding episode, including a joint bleeding episode.
17. Patient Counseling Information
Advise the patients to:
- Read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Call their healthcare provider or go to the emergency department right away if a hypersensitivity reaction occurs. Early signs of hypersensitivity reactions may include rash, hives, itching, facial swelling, tightness of the chest, and wheezing. Advise patients to discontinue use of the product if these symptoms occur and seek immediate emergency treatment.
- Contact their healthcare provider or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to factor VIII therapy because this may be a sign of inhibitor development.
- Consult with their physicians or healthcare provider prior to travel. While traveling, advise patients to bring an adequate supply of ADYNOVATE based on their current regimen of treatment.
To enroll in the confidential, industry-wide Patient Notification System, call 1-888-873-2838.
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ADYNOVATE
antihemophilic factor (recombinant) pegylated kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals Amercia, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BAXALTA US INC. | 009471603 | MANUFACTURE(0944-4622, 0944-4623, 0944-4626, 0944-4624, 0944-4627, 0944-4625, 0944-4628) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Siegfried Hameln GmbH | 315869123 | MANUFACTURE(64764-514, 64764-515) | |