Drug Detail:Afluria (Influenza virus vaccine (injection) [ in-floo-enz-a-vye-rus-vak-seen ])
Drug Class: Viral vaccines
Highlights of Prescribing Information
AFLURIA, Influenza Virus Vaccine
Suspension for Intramuscular Injection
2010-2011 Formula
Initial U.S. Approval: 2007
Recent Major Changes
| Indications and Usage (1) | 11/2009 |
| Dosage and Administration (2.2) | 11/2009 |
| Warnings and Precautions (5.1) | 7/2010 |
Indications and Usage for Afluria
- AFLURIA is an inactivated influenza virus vaccine indicated for active immunization of persons ages 6 months and older against influenza disease caused by influenza virus subtypes A and type B present in the vaccine. (1)
- This indication is based on the immune response elicited by AFLURIA; there have been no controlled clinical studies demonstrating a decrease in influenza disease after vaccination with AFLURIA. (14)
Afluria Dosage and Administration
Children
-
6 months through 35 months of age (0.25 mL dose, intramuscular injection):
Previously unvaccinated children should receive two 0.25 mL doses; one on day 1 followed by another approximately 4 weeks later. (2.2)
Previously vaccinated children should receive only one 0.25 mL dose. (2.2) -
36 months through 8 years of age (0.5 mL dose, intramuscular injection):
Previously unvaccinated children should receive two 0.5 mL doses, one on day 1 followed by another approximately 4 weeks later. (2.2)
Previously vaccinated children should receive only one 0.5 mL dose. (2.2) -
9 years of age and older
A single 0.5 mL dose for intramuscular injection. (2.2)
Adults
A single 0.5 mL dose for intramuscular injection. (2.2)
Dosage Forms and Strengths
AFLURIA, a sterile suspension for intramuscular injection, is supplied in two presentations:
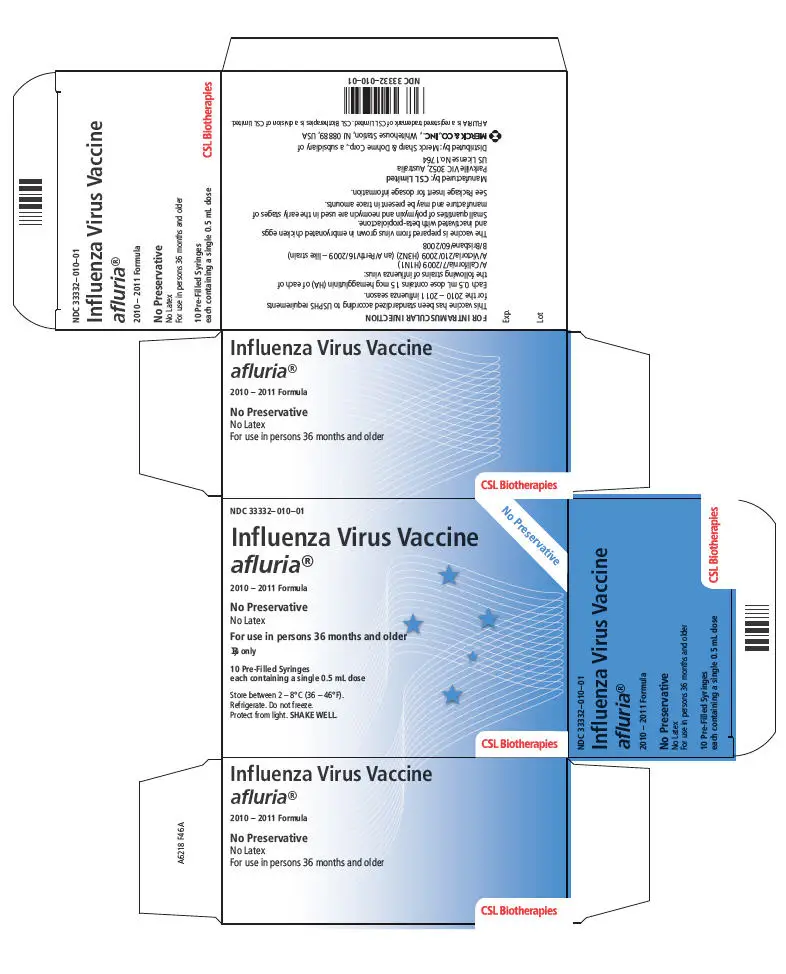
- 0.5 mL single-dose, pre-filled syringe, no preservative. (3)
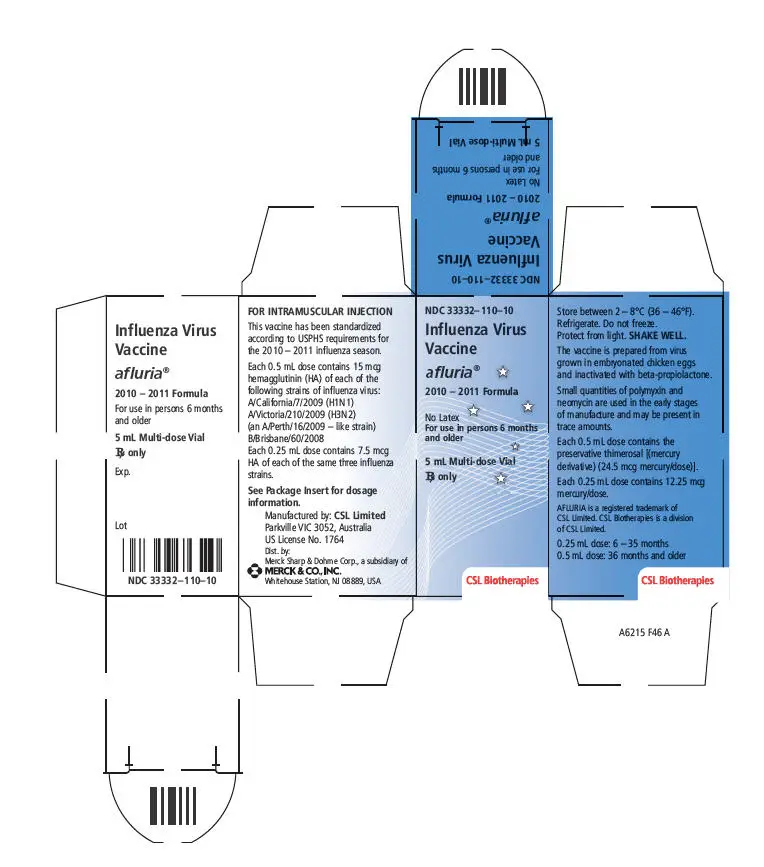
- 5 mL multi-dose vial containing ten 0.5 mL doses. Thimerosal, a mercury derivative, is added as a preservative; each 0.5 mL dose contains 24.5 micrograms (mcg) of mercury. (3,11)
Contraindications
- Hypersensitivity to eggs, neomycin, or polymyxin, or life-threatening reaction to previous influenza vaccination. (4)
Warnings and Precautions
- Administration of CSL's 2010 Southern Hemisphere influenza vaccine has been associated with increased postmarketing reports of fever and febrile seizures in children predominantly below the age of 5 years as compared to previous years. (5.1)
- If Guillain-Barré Syndrome (GBS) has occurred within 6 weeks of previous influenza vaccination, the decision to give AFLURIA should be based on careful consideration of the potential benefits and risks. (5.2)
- Immunocompromised persons may have a diminished immune response to AFLURIA. (5.3)
Adverse Reactions/Side Effects
- In adults, the most common (≥ 10%) local (injection-site) adverse reactions were tenderness, pain, redness, and swelling. The most common (≥ 10%) systemic adverse reactions were headache, malaise, and muscle aches. (6)
- In children, the most common (≥ 10%) local (injection-site) adverse reactions were pain, redness, and swelling. The most common (≥ 10%) systemic adverse reactions were irritability, rhinitis, fever, cough, loss of appetite, vomiting/diarrhea, headache, muscle aches and sore throat. (6)
- Administration of CSL's 2010 Southern Hemisphere influenza vaccine has been associated with increased postmarketing reports of fever and febrile seizures in children predominantly below the age of 5 years as compared to previous years. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. at 1-877-888-4231 or VAERS at 1-800-822-7967 and www.vaers.hhs.gov.
Drug Interactions
- Do not mix with any other vaccine in the same syringe or vial. (7.1)
- Immunosuppressive therapies may diminish the immune response to AFLURIA. (7.2)
Use In Specific Populations
- Safety and effectiveness of AFLURIA have not been established in pregnant women or nursing mothers and in the pediatric population below 6 months of age. (8.1, 8.3, 8.4)
- Antibody responses were lower in geriatric subjects than in younger subjects. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2010
Full Prescribing Information
1. Indications and Usage for Afluria
AFLURIA® is an inactivated influenza virus vaccine indicated for active immunization of persons ages 6 months and older against influenza disease caused by influenza virus subtypes A and type B present in the vaccine.
This indication is based on the immune response elicited by AFLURIA; there have been no controlled clinical studies demonstrating a decrease in influenza disease after vaccination with AFLURIA (see Clinical Studies [14]).
2. Afluria Dosage and Administration
2.1 Prior to Administration
AFLURIA should be inspected visually for particulate matter and discoloration prior to administration (see Description [11]), whenever suspension and container permit. If either of these conditions exists, the vaccine should not be administered. Any vaccine that has been frozen or is suspected of being frozen must not be used.
2.2 Administration
When using a preservative-free, single-dose syringe, shake the syringe thoroughly and administer the dose immediately.
When using the multi-dose vial, shake the vial thoroughly before withdrawing each dose, and administer the dose immediately. Between uses, store the vial at 2–8°C (36–46°F) (see How Supplied/Storage and Handling [16]). Once the stopper has been pierced, the vial must be discarded within 28 days.
3. Dosage Forms and Strengths
AFLURIA is a sterile suspension for intramuscular injection (see Description [11]).
AFLURIA is supplied in two presentations:
- 0.5 mL single-dose, pre-filled syringe, no preservative.
- 5 mL multi-dose vial. Thimerosal, a mercury derivative, is added as a preservative; each 0.5 mL dose contains 24.5 mcg of mercury.
4. Contraindications
AFLURIA is contraindicated in individuals with known hypersensitivity to eggs, neomycin, or polymyxin, or in anyone who has had a life-threatening reaction to previous influenza vaccination (see Description [11]).
5. Warnings and Precautions
5.1 Fever and Febrile Seizures
Administration of CSL's 2010 Southern Hemisphere influenza vaccine has been associated with increased postmarketing reports of fever and febrile seizures in children predominantly below the age of 5 years as compared to previous years.
5.2 Guillain-Barré Syndrome (GBS)
If GBS has occurred within 6 weeks of previous influenza vaccination, the decision to give AFLURIA should be based on careful consideration of the potential benefits and risks.
5.3 Altered Immunocompetence
If AFLURIA is administered to immunocompromised persons, including those receiving immunosuppressive therapy, the immune response may be diminished.
6. Adverse Reactions/Side Effects
6.1 Overall Adverse Reactions
Serious allergic reactions, including anaphylactic shock, have been observed during postmarketing surveillance in individuals receiving AFLURIA. Administration of CSL's 2010 Southern Hemisphere influenza vaccine [formulated to contain A/California/7/2009 (H1N1), A/Wisconsin/15/2009 (H3N2) and B/Brisbane/60/2008 (B Strain)] has been associated with increased postmarketing reports of fever and febrile seizures in children predominantly below the age of 5 years as compared to previous years (see Warnings and Precautions [5.1]).
In adults, the most common local (injection-site) adverse reactions observed in clinical studies with AFLURIA were tenderness, pain, redness (erythema), and swelling. The most common systemic adverse reactions observed were headache, malaise, and muscle aches (myalgia).
In children, the most common local (injection-site) adverse reactions observed in a clinical study with AFLURIA were pain, redness and swelling. The most common systemic adverse reactions observed were irritability, rhinitis, fever, cough, loss of appetite, vomiting/diarrhea, headache, muscle aches and sore throat.
6.2 Safety Experience from Clinical Studies
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a vaccine cannot be directly compared to rates in the clinical studies of another vaccine and may not reflect the rates observed in clinical practice.
Clinical data for AFLURIA have been obtained in four clinical studies, three in adult populations (Studies 1 to 3) and one in a pediatric population (Study 4) (see Clinical Studies [14]). Clinical safety data are provided for two of the adult studies (Studies 1 and 2) and one pediatric study (Study 4). Rates of solicited fever in children from a second pediatric study (Study 5) are also provided.
A US study (Study 1) included 1,357 subjects for safety analysis, ages 18 to less than 65 years, randomized to receive AFLURIA (1,089 subjects) or placebo (268 subjects) (see Clinical Studies [14] for study demographics). There were no deaths or serious adverse events reported in this study.
A UK study (Study 2) included 275 subjects, ages 65 years and older, randomized to receive preservative-free AFLURIA (206 subjects) or a European-licensed trivalent inactivated influenza vaccine as an active control (69 subjects) (see Clinical Studies [14]). There were no deaths or serious adverse events reported in this study.
An open-label, uncontrolled study in children, conducted in Australia (Study 4), included 298 subjects, ages 6 months to less than 9 years. All subjects received preservative-free AFLURIA administered as two doses, one month apart (see Clinical Studies [14]). Subjects were subdivided into two age groups: children ages 6 months to less than 3 years (151 subjects) received two 0.25 mL doses of AFLURIA and children ages 3 years to less than 9 years (147 subjects) received two 0.5 mL doses of AFLURIA. There were no deaths or vaccine-related serious adverse events reported in this study.
The safety assessment was identical for the two adult studies. Local (injection-site) and systemic adverse events were solicited by completion of a symptom diary card for 5 days post-vaccination (Table 1). Unsolicited adverse events were collected for 21 days post-vaccination (Table 2). These unsolicited adverse events were reported either spontaneously or when subjects were questioned about any changes in their health post-vaccination. All adverse events are presented regardless of any treatment causality assigned by study investigators.
In the open-label pediatric study (Study 4), solicited adverse events were recorded for up to 7 days (Table 3) and unsolicited adverse events were recorded for 30 days post-vaccination (Table 4). Data are presented following each dose for each age group. All adverse events are presented regardless of any treatment causality assigned by study investigators.
Rates of solicited fever in the seven days following vaccination with the 2009-2010 formulation of AFLURIA or another U.S. licensed influenza vaccine (manufactured by Sanofi Pasteur, Inc.) in children 6 months to less than 18 years of age (Study 5) are shown in Table 5.
| Study 1 Subjects ≥ 18 to < 65 years | Study 2 Subjects ≥ 65 years |
||
|---|---|---|---|
| Solicited Adverse Event | AFLURIA‡
n=1089 | Placebo§
n=268 | AFLURIA n=206 |
|
|||
| Local | |||
| Tenderness¶ | 60% | 18% | 34% |
| Pain# | 40% | 9% | 9% |
| Redness | 16% | 8% | 23% |
| Swelling | 9% | 1% | 11% |
| Bruising | 5% | 1% | 4% |
| Systemic | |||
| Headache | 26% | 26% | 15% |
| Malaise | 20% | 19% | 10% |
| Muscle aches | 13% | 9% | 14% |
| Nausea | 6% | 9% | 3% |
| Chills/Shivering | 3% | 2% | 7% |
| Fever ≥ 37.7°C (99.9°F) | 1% | 1% | 1% |
| Vomiting | 1% | 1% | 0% |
| Study 1 Subjects ≥ 18 to < 65 years | Study 2 Subjects ≥ 65 years |
||
|---|---|---|---|
| Adverse Event | AFLURIA‡
n=1089 | Placebo§
n=268 | AFLURIA n=206 |
|
|||
| Headache | 8% | 6% | 8% |
| Nasal Congestion | 1% | 1% | 7% |
| Cough | 1% | 0.4% | 5% |
| Rhinorrhea | 1% | 1% | 5% |
| Pharyngolaryngeal Pain | 3% | 1% | 5% |
| Reactogenicity Event | 3% | 3% | 0% |
| Diarrhea | 2% | 3% | 1% |
| Back Pain | 2% | 0.4% | 2% |
| Upper Respiratory Tract Infection | 2% | 1% | 0.5% |
| Viral Infection | 0.4% | 1% | 0% |
| Lower Respiratory Tract Infection | 0% | 0% | 1% |
| Myalgia | 1% | 1% | 1% |
| Muscle Spasms | 0.4% | 1% | 0% |
| Subjects ≥ 6 months to < 3 years (n = 151)‡ | Subjects ≥ 3 years to < 9 years (n = 147)§ |
|||
|---|---|---|---|---|
| Solicited Adverse Event | Dose 1 | Dose 2 | Dose 1 | Dose 2 |
|
||||
| Local | ||||
| Pain | 36% | 37% | 59% | 62% |
| Erythema | 36% | 38% | 37% | 46% |
| Swelling | 16% | 21% | 25% | 27% |
| Systemic | ||||
| Irritability | 48% | 41% | 20% | 17% |
| Rhinitis | 37% | 48% | 21% | 29% |
| Fever¶ | 23% | 23% | 16% | 8% |
| Cough | 21% | 32% | 19% | 19% |
| Loss of appetite | 19% | 24% | 8% | 5% |
| Vomiting/Diarrhea | 15% | 14% | 8% | 7% |
| Headache | 2%# | 3%Þ | 14% | 11% |
| Myalgia | 1%ß | 3%Þ | 14% | 8% |
| Sore throat | 2%# | 5%Þ | 8% | 11% |
| Wheezing/Shortness of breath | 3% | 9% | 3% | 2% |
| Ear ache | 3%Þ | 3%ß | 4% | 1% |
| Subjects ≥ 6 months to < 3 years (n = 151)† | Subjects ≥ 3 to < 9 years (n = 147)‡ |
|||
|---|---|---|---|---|
| Adverse Event | Dose 1 | Dose 2 | Dose 1 | Dose 2 |
|
||||
| Nasopharyngitis | 5.3% | 7.9% | 5.4% | 5.4% |
| Rhinitis | 13.2% | 9.9% | 6.8% | 10.9% |
| Upper Respiratory Tract Infection | 9.9% | 7.3% | 6.1% | 6.1% |
| Irritability | 3.3% | 5.3% | 0.7% | 0.7% |
| Headache | 1.3% | 0.7% | 6.1% | 4.1% |
| Cough | 10.6% | 13.2% | 10.9% | 13.6% |
| Rhinorrhea | 7.3% | 6.0% | 6.8% | 4.8% |
| Teething | 14.6% | 9.9% | 0.0% | 0.0% |
| Vomiting | 5.3% | 2.6% | 2.0% | 2.7% |
| Influenza-like Illness | 13.9% | 10.6% | 6.8% | 3.4% |
| Pyrexia | 2.6% | 9.3% | 2.7% | 4.1% |
| Age Group | |||||||
|---|---|---|---|---|---|---|---|
| 6 months to < 3 years† | 3 to < 5 years‡ | 5 to < 9 years§ | 9 to < 18 years¶ | ||||
| Dose 1 | Dose 2 | Dose 1 | Dose 2 | Dose 1 | Dose 2 | Dose 1 | |
|
|||||||
| AFLURIA# | 37% | 15% | 32% | 14% | 16% | 0% | 6% |
| Comparator# | 14% | 14% | 11% | 16% | 9% | 2% | 4% |
6.3 Postmarketing Experience
Because postmarketing reporting of adverse reactions is voluntary and from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure. The adverse reactions described have been included in this section because they: 1) represent reactions that are known to occur following immunizations generally or influenza immunizations specifically; 2) are potentially serious; or 3) have been reported frequently. These adverse reactions reflect experience in both children and adults and include those identified during post-approval use of AFLURIA outside the US since 1985.
Blood and lymphatic system disorders
Transient thrombocytopenia
Immune system disorders
Allergic reactions including anaphylactic shock and serum sickness
Nervous system disorders
Neuralgia, paresthesia, and convulsions (including febrile seizures); encephalopathy, neuritis or neuropathy, transverse myelitis, and GBS
Vascular disorders
Vasculitis with transient renal involvement
Skin and subcutaneous tissue disorders
Pruritus, urticaria, and rash
6.4 Other Adverse Reactions Associated With Influenza Vaccination
Anaphylaxis has been reported after administration of AFLURIA. Egg protein can induce immediate hypersensitivity reactions among persons who have severe egg allergy. Allergic reactions include hives, angioedema, asthma, and systemic anaphylaxis (see Contraindications [4]).
The 1976 swine influenza vaccine was associated with an increased frequency of GBS. Evidence for a causal relation of GBS with subsequent vaccines prepared from other influenza viruses is unclear. If influenza vaccine does pose a risk, it is probably slightly more than one additional case per 1 million persons vaccinated.
Neurological disorders temporally associated with influenza vaccination, such as encephalopathy, optic neuritis/neuropathy, partial facial paralysis, and brachial plexus neuropathy, have been reported.
Microscopic polyangiitis (vasculitis) has been reported temporally associated with influenza vaccination.
7. Drug Interactions
7.1 Concurrent Use With Other Vaccines
There are no data to assess the concomitant administration of AFLURIA with other vaccines. If AFLURIA is to be given at the same time as another injectable vaccine(s), the vaccine(s) should be administered at different injection sites.
AFLURIA should not be mixed with any other vaccine in the same syringe or vial.
8. Use In Specific Populations
8.3 Nursing Mothers
AFLURIA has not been evaluated in nursing mothers. It is not known whether AFLURIA is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when AFLURIA is administered to a nursing woman.
8.4 Pediatric Use
Safety and effectiveness of AFLURIA in children below 6 months of age have not been established. The safety and immunogenicity of AFLURIA was evaluated in 298 children between the ages of 6 months and 9 years (Study 4). In this study the incidence of fever in children 6 months to < 3 years of age following the first and second doses of AFLURIA was 23%. Among children 3 years to < 9 years of age the incidence was 16% following the first dose and 8% following the second dose. Administration of CSL's 2010 Southern Hemisphere influenza vaccine has been associated with increased postmarketing reports of fever and febrile seizures in children predominantly below the age of 5 years as compared to previous years (see Adverse Reactions [6.2] and Warnings and Precautions [5.1]).
8.5 Geriatric Use
In four clinical studies, 343 subjects ages 65 years and older received AFLURIA. Hemagglutination-inhibiting antibody responses in geriatric subjects were lower after administration of AFLURIA in comparison to younger adult subjects (see Clinical Studies [14]). Adverse event rates were generally similar in frequency to those reported in subjects ages 18 to less than 65 years, although some differences were observed (see Adverse Reactions [6.2]).
11. Afluria Description
AFLURIA, Influenza Virus Vaccine for intramuscular injection, is a sterile, clear, colorless to slightly opalescent suspension with some sediment that resuspends upon shaking to form a homogeneous suspension. AFLURIA is prepared from influenza virus propagated in the allantoic fluid of embryonated chicken eggs. Following harvest, the virus is purified in a sucrose density gradient using a continuous flow zonal centrifuge. The purified virus is inactivated with beta-propiolactone, and the virus particles are disrupted using sodium taurodeoxycholate to produce a "split virion". The disrupted virus is further purified and suspended in a phosphate buffered isotonic solution.
AFLURIA is standardized according to USPHS requirements for the 2010-2011 influenza season and is formulated to contain 45 mcg hemagglutinin (HA) per 0.5 mL dose in the recommended ratio of 15 mcg HA for each of the three influenza strains recommended for the 2010-2011 Northern Hemisphere influenza season: A/California/7/2009, NYMC X-181 (H1N1), A/Victoria/210/2009, NYMC X-187 (H3N2) (an A/Perth/16/2009-like strain), and B/Brisbane/60/2008. A 0.25 mL dose contains 7.5 mcg HA of each of the same three influenza strains.
Thimerosal, a mercury derivative, is not used in the manufacturing process for the single dose presentations; therefore these products contain no preservative. The multi-dose presentation contains thimerosal, added as a preservative; each 0.5 mL dose contains 24.5 mcg of mercury.
A single 0.5 mL dose of AFLURIA contains sodium chloride (4.1 mg), monobasic sodium phosphate (80 mcg), dibasic sodium phosphate (300 mcg), monobasic potassium phosphate (20 mcg), potassium chloride (20 mcg), and calcium chloride (1.5 mcg). From the manufacturing process, each 0.5 mL dose may also contain residual amounts of sodium taurodeoxycholate (≤ 10 ppm), ovalbumin (≤ 1 mcg), neomycin sulfate (≤ 0.2 picograms [pg]), polymyxin B (≤ 0.03 pg), and beta-propiolactone (< 25 nanograms). A single 0.25 mL dose of AFLURIA contains half of these quantities.
The rubber tip cap and plunger used for the preservative-free, single-dose syringes and the rubber stoppers used for the multi-dose vial contain no latex.
12. Afluria - Clinical Pharmacology
12.1 Mechanism of Action
Influenza illness and its complications follow infection with influenza viruses. Global surveillance of influenza identifies yearly antigenic variants. For example, since 1977 antigenic variants of influenza A (H1N1 and H3N2) and influenza B viruses have been in global circulation. Specific levels of hemagglutination inhibition (HI) antibody titers post-vaccination with inactivated influenza virus vaccine have not been correlated with protection from influenza virus. In some human studies, antibody titers of 1:40 or greater have been associated with protection from influenza illness in up to 50% of subjects.2,3
Antibody against one influenza virus type or subtype confers limited or no protection against another. Furthermore, antibody to one antigenic variant of influenza virus might not protect against a new antigenic variant of the same type or subtype. Frequent development of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and the reason for the usual change to one or more new strains in each year's influenza vaccine. Therefore, inactivated influenza vaccines are standardized to contain the HA of three strains (i.e., typically two type A and one type B) representing the influenza viruses likely to be circulating in the US during the upcoming winter.
Annual revaccination with the current vaccine is recommended because immunity declines during the year after vaccination and circulating strains of influenza virus change from year to year.1
14. Clinical Studies
14.1 Immunogenicity in the Adult and Geriatric Populations
Three randomized, controlled clinical studies of AFLURIA have evaluated the immune responses by measuring HI antibody titers to each virus strain in the vaccine. In these studies, post-vaccination immunogenicity was evaluated on sera obtained 21 days after administration of AFLURIA. No controlled clinical studies demonstrating a decrease in influenza disease after vaccination with AFLURIA have been performed.
The US study (Study 1) was a randomized, double-blinded, placebo-controlled, multicenter study in healthy subjects ages 18 to less than 65 years. A total of 1,357 subjects were vaccinated (1,089 subjects with AFLURIA and 268 with a thimerosal-containing placebo). Subjects receiving AFLURIA were vaccinated using either a single-dose (preservative-free) or multi-dose (one of three lots) formulation. The evaluable efficacy population consisted of 1,341 subjects (1,077 in the AFLURIA group and 264 in the placebo group) with complete serological data who had not received any contraindicated medications before the post-vaccination immunogenicity assessment. Among the evaluable efficacy population receiving AFLURIA, 37.5% were men and 62.5% were women. The mean age of the entire evaluable population receiving AFLURIA was 38 years; 73% were ages 18 to less than 50 years and 27% were ages 50 to less than 65 years. Additionally, 81% of AFLURIA recipients were White, 12% Black, and 6% Asian.
In Study 1, the following co-primary immunogenicity endpoints were assessed: 1) the lower bounds of the 2-sided 95% confidence intervals (CI) for the proportion of subjects with HI antibody titers of 1:40 or greater after vaccination, which should exceed 70% for each vaccine antigen strain; and 2) the lower bounds of the 2-sided 95% CI for rates of seroconversion (defined as a 4-fold increase in post-vaccination HI antibody titers from pre-vaccination titers of 1:10 or greater, or an increase in titers from less than 1:10 to 1:40 or greater), which should exceed 40% for each vaccine antigen strain.
In subjects ages 18 to less than 65 years, serum HI antibody responses to AFLURIA met the pre-specified co-primary endpoint criteria for all three virus strains (Table 6). Clinical lot-to-lot consistency was demonstrated for the single-dose (preservative-free) and multi-dose formulations of AFLURIA, showing that these formulations elicited similar immune responses.
| Treatment Arm | Number Enrolled/Evaluable | Vaccine Strain | Seroconversion Rate*
(95% CI) | HI Titer ≥ 1:40†
(95% CI) |
|---|---|---|---|---|
|
||||
| All active AFLURIA influenza vaccine formulations‡ | 1089/1077 | H1N1 | 48.7% (45.6, 51.7) | 97.8% (96.7, 98.6) |
| H3N2 | 71.5% (68.7, 74.2) | 99.9% (99.5, 100.0) |
||
| B | 69.7% (66.9, 72.5) | 94.2% (92.7, 95.6) |
||
| Placebo | 270/264 | H1N1 | 2.3% (0.8, 4.9) | 74.6% (68.9, 79.8) |
| H3N2 | 0.0% (N/A) | 72.0% (66.1, 77.3) |
||
| B | 0.4% (< 0.1, 2.1) | 47.0% (40.8, 53.2) |
||
The UK study (Study 2) was a randomized, controlled study that enrolled 275 healthy subjects ages 65 years and older. This study compared AFLURIA with a European-licensed trivalent inactivated influenza vaccine as an active control. The evaluable efficacy population consisted of 274 subjects (206 in the AFLURIA group and 68 in the control group). Among these subjects, 50% were men and 50% were women, with a mean age of 72 years (range: 65 to 93 years).
The co-primary immunogenicity endpoints for the seroconversion rate and the proportion of subjects with a minimum post-vaccination HI antibody titer of 1:40 are presented in Table 7.
| Number of Subjects | Vaccine Strain | Seroconversion Rate*
(95% CI) | HI Titer ≥ 1:40†
(95% CI) |
|---|---|---|---|
|
|||
| 206 | H1N1 | 34.0% (27.5, 40.9) | 85.0% (79.3, 89.5) |
| H3N2 | 44.2% (37.3, 51.2) | 99.5% (97.3, 100.0) | |
| B | 45.6% (38.7, 52.7) | 77.7% (71.4, 83.2) | |
A second UK study (Study 3) was a randomized, controlled study that enrolled 406 healthy subjects ages 18 years and older (stratified by age from 18 to less than 60 years and 60 years and older). This study compared AFLURIA with a European-licensed trivalent inactivated influenza vaccine as an active control. In a post-hoc analysis of different age ranges, among subjects ages 18 to less than 65 years receiving AFLURIA (146 subjects), 47% were men and 53% were women, with a mean age of 48 years for all subjects. Among subjects ages 65 years and older receiving AFLURIA (60 subjects), 53% were men and 47% were women, with a mean age of 71 years.
Analysis of serum HI antibody responses showed that the lower bound of the 95% CI for subjects with HI antibody titers of 1:40 or greater after vaccination exceeded 70% for each strain. HI antibody responses were lower in subjects, ages 65 years and older after administration of AFLURIA. Serum HI antibody responses to the active control were similar to those for AFLURIA in both age groups.
14.2 Immunogenicity in a Pediatric Population
An open-label, uncontrolled, multi-center study (Study 4) to evaluate the safety, tolerability and immunogenicity of AFLURIA in children 6 months to 9 years of age was conducted in Australia. The study subjects were subdivided into two groups dependent upon age at time of enrollment. A total of 298 subjects were enrolled, including 151 subjects, 6 months to less than 3 years (mean age 1.7 years with 51.0% females) and 147 subjects, 3 years to less than 9 years (mean age 5 years with 55.1% females).
Two doses of AFLURIA were administered to all subjects, with a 30 day interval between each dose. Children ages 6 months to less than 3 years received two 0.25 mL doses of AFLURIA. Children ages 3 years to less than 9 years were administered two 0.5 mL doses of AFLURIA. Sera for immunological assessment were taken 30 days (± 3) following each vaccination. Immunogenicity endpoints were the seroconversion rate and the proportion of subjects with a minimum post-vaccination HI antibody titer of 1:40. The results for each dose are presented in Table 8.
For both age groups, the vaccine met FDA acceptance criteria for immunogenicity developed for healthy adults for all three influenza strains following two doses. These criteria are: 1) that the lower bound of the 2-sided 95% CI for the seroconversion rate should be at least 40%; and 2) the lower bound of the 2-sided 95% CI for the proportion of subjects with a post-vaccination HI titer of ≥ 1:40 should be at least 70%.
| Vaccine Strain | Vaccine Dose | Seroconversion Rate*
(lower 95% CI) | HI Titer ≥ 1:40†
(lower 95% CI) |
|
|---|---|---|---|---|
|
||||
| Subjects ≥ 6 months to < 3 years n=143‡ n=139§ | H1N1 | Dose 1 | 16.1% (> 11.3) | 16.1% (> 11.3) |
| Dose 2 | 95.0% (> 90.8) | 95.7% (> 91.7) | ||
| H3N2 | Dose 1 | 86.0% (> 80.3) | 97.9% (> 94.7) | |
| Dose 2 | 90.6% (> 85.6) | 100.0% (> 97.9) | ||
| B | Dose 1 | 20.3% (> 14.9) | 21.0% (> 15.5) | |
| Dose 2 | 94.2% (> 89.9) | 95.7% (> 91.7) | ||
| Subjects
≥ 3 years to < 9 years n=144‡ n=132§ | H1N1 | Dose 1 | 24.3% (> 18.5) | 25.7% (> 19.8) |
| Dose 2 | 93.9% (> 89.3) | 95.5% (> 91.2) | ||
| H3N2 | Dose 1 | 68.1% (> 61.1) | 98.6% (> 95.7) | |
| Dose 2 | 70.5% (> 63.2) | 100.0% (> 97.8) | ||
| B | Dose 1 | 32.6% (> 26.2) | 34.0% (> 27.5) | |
| Dose 2 | 93.2% (> 88.4) | 94.7% (> 90.3) | ||
15. References
- Centers for Disease Control and Prevention. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009;58 (RR-8):1-46.
- Hannoun C, Megas F, Piercy J. Immunogenicity and Protective Efficacy of Influenza Vaccination. Virus Res 2004;103:133-138.
- Hobson D, Curry RL, Beare AS, et al. The Role of Serum Hemagglutination-Inhibiting Antibody in Protection Against Challenge Infection with Influenza A2 and B Viruses. J Hyg Camb 1972;70:767-777.
16. How is Afluria supplied
| How Supplied | NDC Number |
|---|---|
| Package of ten 0.5 mL single-dose, prefilled syringes without needles | 33332-010-01 |
| Package of one 5 mL multi-dose vial, which contains ten 0.5 mL doses | 33332-110-10 |
17. Patient Counseling Information
- Inform the patient that AFLURIA is an inactivated vaccine that cannot cause influenza but stimulates the immune system to produce antibodies that protect against influenza. The full effect of the vaccine is generally achieved approximately 3 weeks after vaccination. Annual revaccination is recommended.
- Instruct the patient to report any severe or unusual adverse reactions to their healthcare provider.
| AFLURIA
influenza a virus a/california/7/2009 x-181 (h1n1) hemagglutinin antigen (propiolactone inactivated), influenza b virus b/brisbane/60/2008 antigen (propiolactone inactivated), and influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (propiolactone inactivated) injection, suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| AFLURIA
influenza a virus a/california/7/2009 x-181 (h1n1) hemagglutinin antigen (propiolactone inactivated), influenza b virus b/brisbane/60/2008 antigen (propiolactone inactivated), and influenza a virus a/victoria/210/2009 x-187 (h3n2) antigen (propiolactone inactivated) injection, suspension |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - CSL Limited (753243823) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring GmbH | 326530474 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Limited | 753243823 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring LLC | 931896963 | MANUFACTURE | |