Drug Detail:Afstyla (recombinant) (Antihemophilic factor (recombinant) [ ant-ee-hee-moe-fil-ik-fak-tor ])
Drug Class: Miscellaneous coagulation modifiers
Highlights of Prescribing Information
AFSTYLA® [Antihemophilic Factor (Recombinant), Single Chain]
Lyophilized Powder for Solution for Intravenous Injection
Initial U.S. Approval: 2016
Indications and Usage for Afstyla
AFSTYLA, Antihemophilic Factor (Recombinant), Single Chain, is a recombinant, antihemophilic factor indicated in adults and children with hemophilia A (congenital Factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes,
- Routine prophylaxis to reduce the frequency of bleeding episodes,
- Perioperative management of bleeding.
Limitation of Use
AFSTYLA is not indicated for the treatment of von Willebrand disease (1).
Afstyla Dosage and Administration
For intravenous use after reconstitution only.
- Each vial of AFSTYLA is labeled with the amount of recombinant Factor VIII in international units (IU or unit). One unit per kilogram body weight will raise the Factor VIII level by 2 IU/dL. (2.1)
- Plasma Factor VIII levels can be monitored using either a chromogenic assay or a one-stage clotting assay – routinely used in US clinical laboratories. If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient's Factor VIII activity level. (2.1, 5.3)
Calculating Required Dose: (2.1)
Dose (IU) = Body Weight (kg) × Desired Factor VIII Rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
Routine Prophylaxis: (2.1)
- Adults and adolescents (≥12 years): The recommended starting regimen is 20 to 50 IU per kg of AFSTYLA administered 2 to 3 times weekly.
- Children (<12 years): The recommended starting regimen is 30 to 50 IU per kg of AFSTYLA administered 2 to 3 times weekly. More frequent or higher doses may be required in children <12 years of age to account for the higher clearance in this age group.
- The regimen may be adjusted based on patient response.
Perioperative Management: (2.1)
Ensure the appropriate Factor VIII activity level is achieved and maintained.
Dosage Forms and Strengths
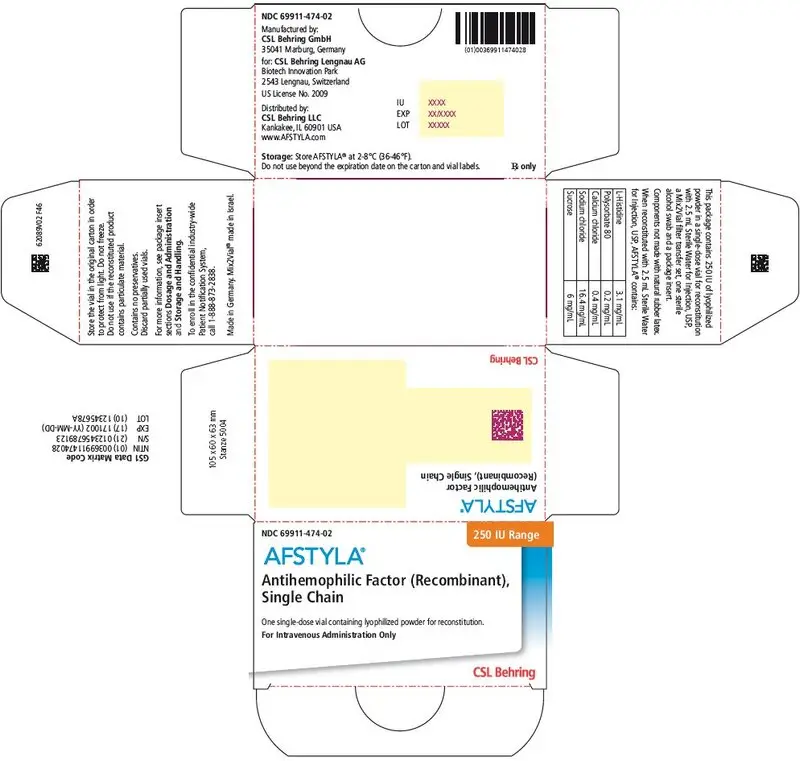
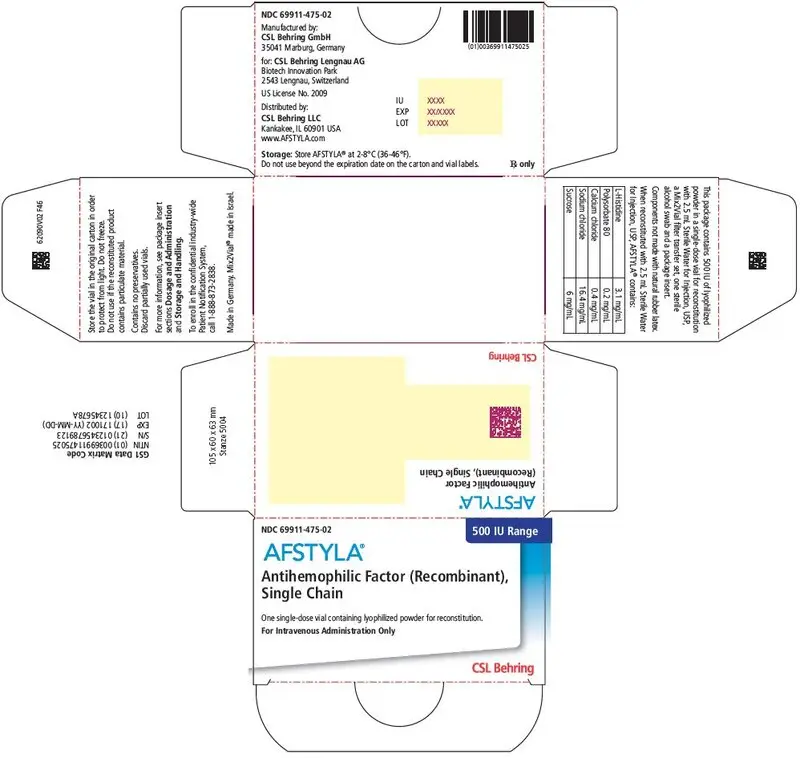
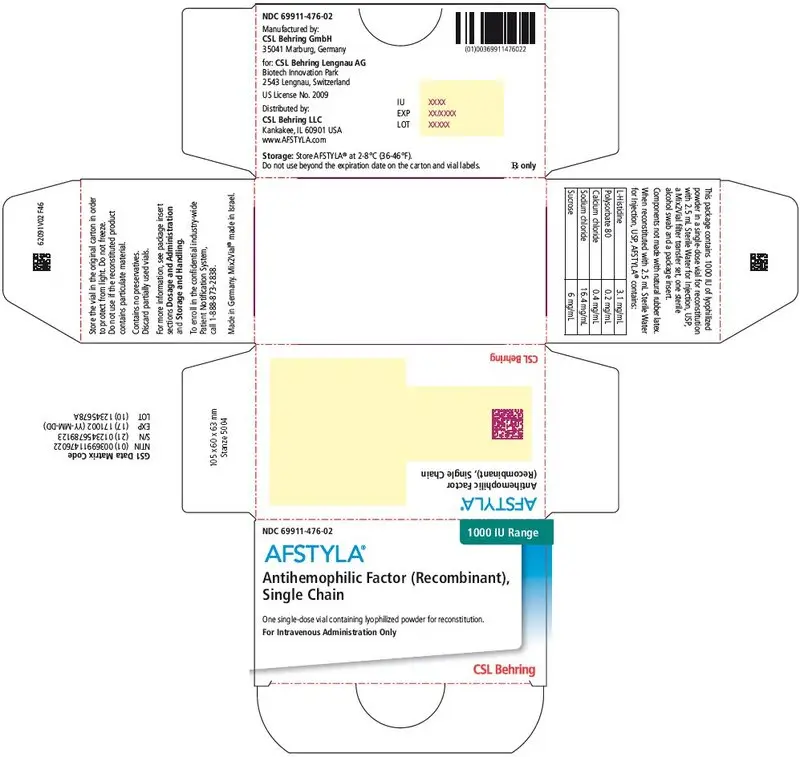
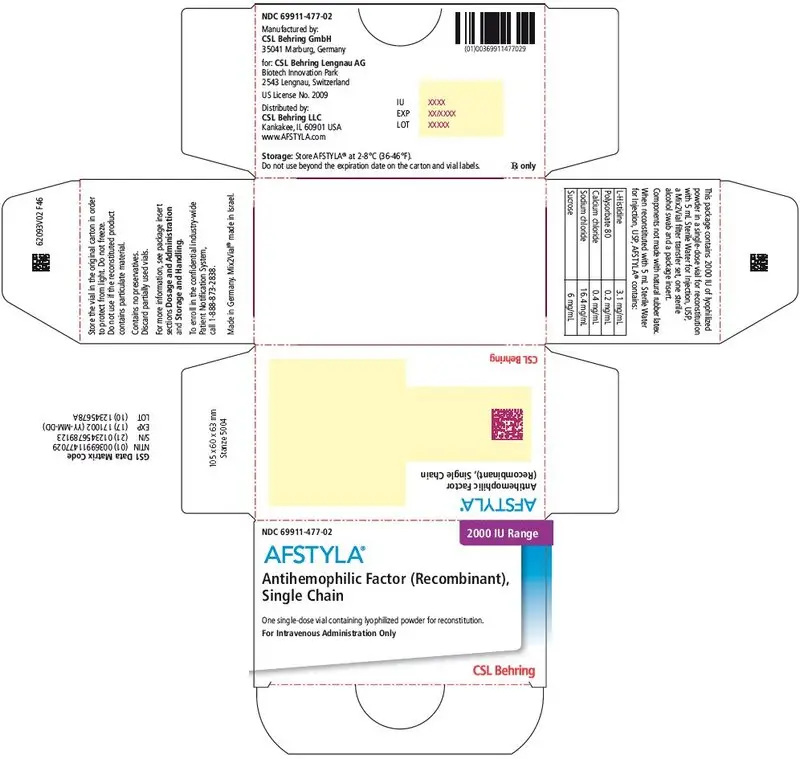
AFSTYLA is available as a white or slightly yellow lyophilized powder supplied in single-dose vials containing nominally 250, 500, 1000, 1500, 2000, 2500, or 3000 International Units (IU). (3)
Contraindications
Do not use in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis to AFSTYLA or its excipients, or hamster proteins. (4)
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, are possible. Should symptoms occur, immediately discontinue AFSTYLA and administer appropriate treatment. (5.1)
- Formation of neutralizing antibodies (inhibitors) to Factor VIII has been reported following administration of AFSTYLA; previously untreated patients (PUPs) are at greater risk.. If expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures Factor VIII inhibitor concentration. (5.2)
- If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient's Factor VIII activity level. (5.3)
Adverse Reactions/Side Effects
- The most common adverse reactions reported in clinical trials (>0.5% of subjects) were Factor VIII inhibition in previously untreated patients (PUPs), dizziness and hypersensitivity. (6)
To report SUSPECTED ADVERSE REACTIONS, contact the CSL Behring Pharmacovigilance Department at 1-866-915-6958 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Pediatric: Clearance (based on per kg body weight) is higher in pediatric patients 0 to <12 years of age. Higher and/or more frequent dosing may be needed. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2023
Full Prescribing Information
1. Indications and Usage for Afstyla
AFSTYLA, Antihemophilic Factor (Recombinant), Single Chain is a recombinant, antihemophilic factor indicated in adults and children with hemophilia A (congenital Factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes,
- Routine prophylaxis to reduce the frequency of bleeding episodes,
- Perioperative management of bleeding.
2. Afstyla Dosage and Administration
For intravenous use after reconstitution only.
2.1 Dosing Guidelines
- Dose and duration of treatment depend on the severity of the Factor VIII deficiency, the location and extent of bleeding, and the patient's clinical condition.
- Each vial of AFSTYLA states the actual amount of Factor VIII activity in International Units (IU) as determined by chromogenic assay. One IU corresponds to the activity of Factor VIII contained in 1 milliliter (mL) of normal human plasma.
- Plasma Factor VIII levels can be monitored using either a chromogenic assay or a one-stage clotting assay – routinely used in US clinical laboratories. If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient's Factor VIII activity level [see Warnings and Precautions (5.3)].
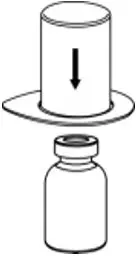
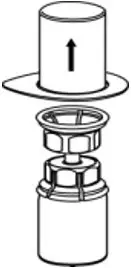
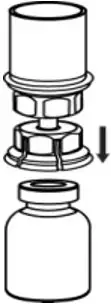
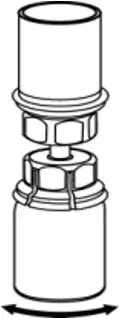
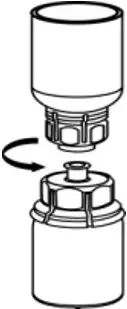
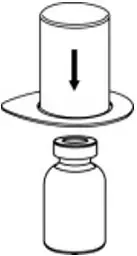
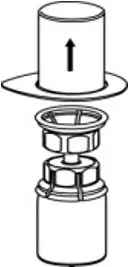
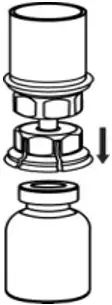
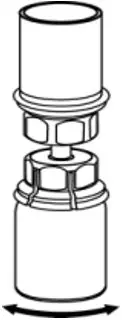
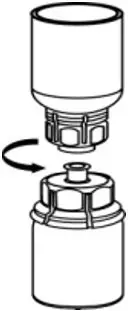
2.2 Preparation and Reconstitution
- Reconstitute AFSTYLA using aseptic technique with diluent provided in the kit.
- Visually inspect the reconstituted solution for particulate matter prior to administration. The solution should be free from visible particles. Do not use if particulate matter is observed.
The procedures provided below are general guidelines for the preparation and reconstitution of AFSTYLA.
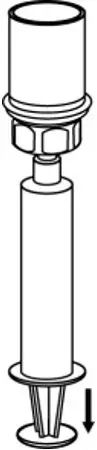
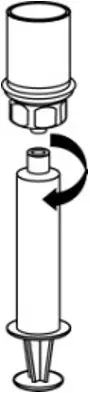
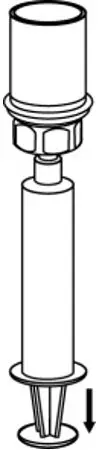
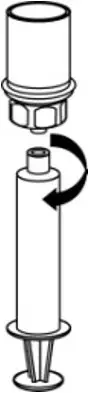
2.3 Administration
- Use aseptic technique when administering AFSTYLA.
- Do not mix AFSTYLA with other medicinal products.
- Administer by intravenous injection. The rate of administration should be determined by the patient's comfort level. Do not exceed infusion rate of 10 mL per minute.
- Administer AFSTYLA at room temperature within 4 hours after reconstitution.
- AFSTYLA is for single-dose only. Following administration, discard any unused solution and all administration equipment in an appropriate manner as per local requirements.
- If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteremia and catheter site thrombosis should be considered.
3. Dosage Forms and Strengths
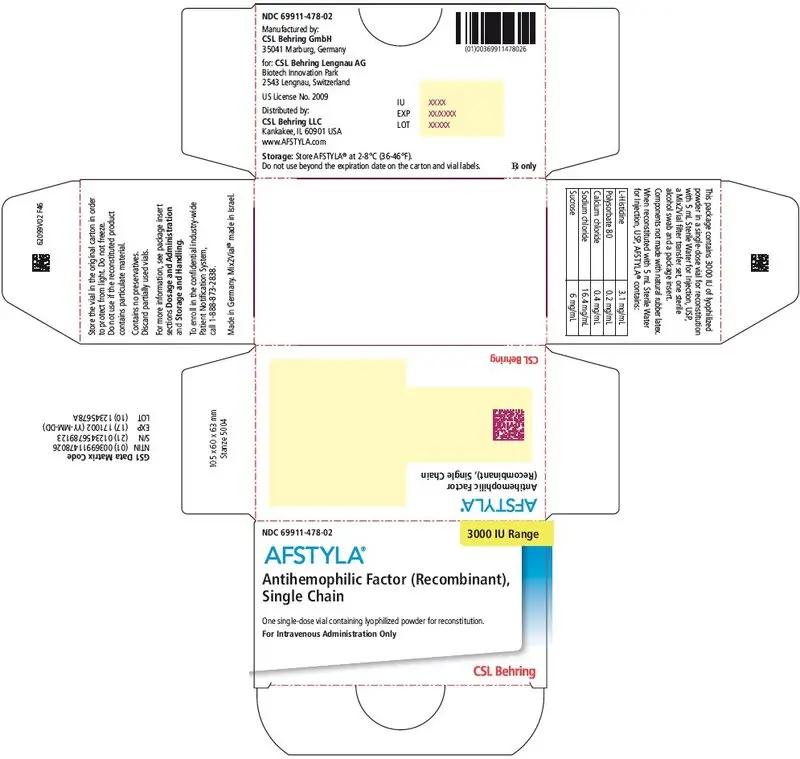
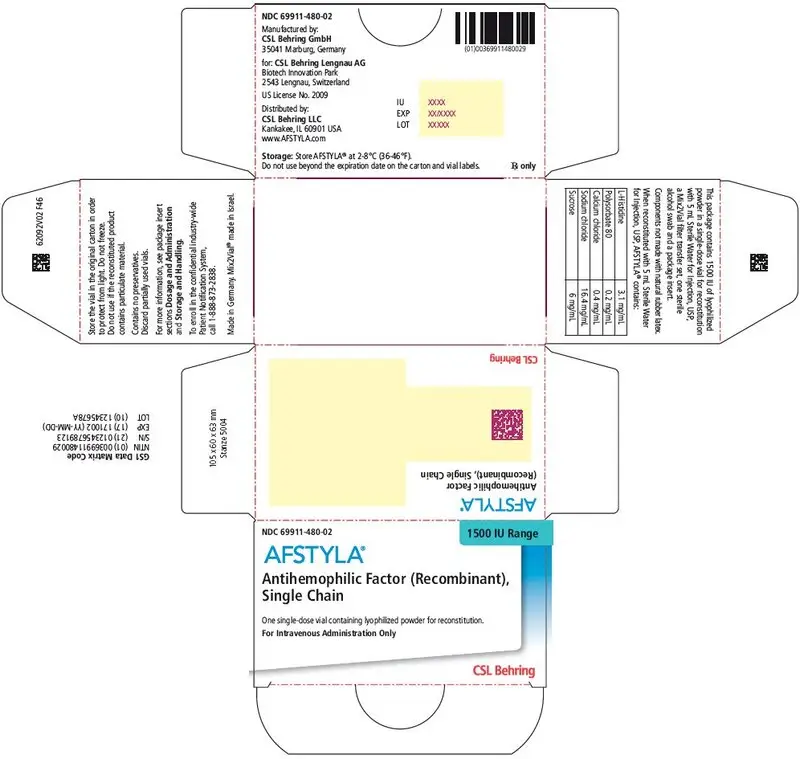
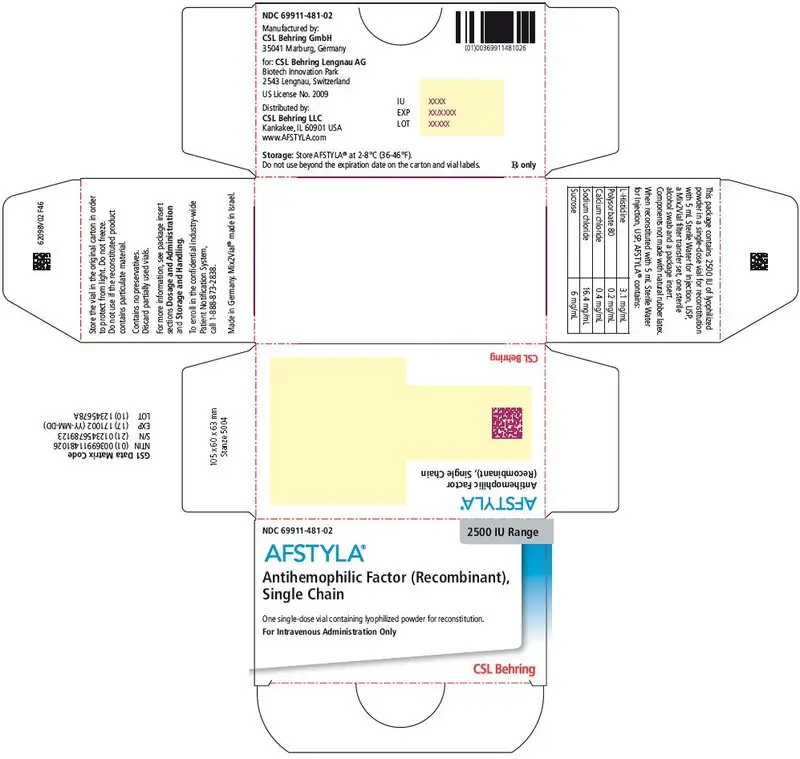
AFSTYLA is available as a white or slightly yellow lyophilized powder supplied in single-dose vials containing nominally 250, 500, 1000, 1500, 2000, 2500, or 3000 IU. The actual potency is labeled on each AFSTYLA vial and carton.
4. Contraindications
AFSTYLA is contraindicated in patients who have had life-threatening hypersensitivity reactions, including anaphylaxis to AFSTYLA or its excipients (e.g., polysorbate 80) [see Description (11)], or hamster proteins [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, are possible with AFSTYLA. Inform patients of the early signs of hypersensitivity reactions that may progress to anaphylaxis (including hives, generalized urticaria, tightness of the chest, wheezing, hypotension and pruritus). Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur.
For patients with previous hypersensitivity reactions, consider premedication with antihistamines.
5.2 Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to Factor VIII has been reported following administration of AFSTYLA; previously untreated patients (PUPs) are at greatest risk [see Adverse Reactions (6.2)]. Monitor patients for the development of neutralizing antibodies (inhibitors) by appropriate clinical observations and laboratory tests. If expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled after AFSTYLA administration, the presence of an inhibitor (neutralizing antibody) should be suspected [see Warnings and Precautions (5.3)].
Contact a specialized hemophilia treatment center if a patient develops an inhibitor.
5.3 Monitoring Laboratory Tests
- Monitor plasma Factor VIII activity in patients receiving AFSTYLA using either the chromogenic assay or the one-stage clotting assay, which is routinely used in US clinical laboratories. The chromogenic assay result most accurately reflects the clinical hemostatic potential of AFSTYLA and is preferred. The one-stage clotting assay result underestimates the Factor VIII activity level compared to the chromogenic assay result by approximately one-half. If the one-stage clotting assay is used, multiply the result by a conversion factor of 2 to determine the patient's Factor VIII activity level. Incorrect interpretation of the Factor VIII activity obtained by the one-stage clotting assay could lead to unnecessary additional dosing, higher chronic dosing, or investigations for an inhibitor.
- Monitor for the development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected plasma Factor VIII activity levels are not attained or if bleeding is not controlled with the expected dose of AFSTYLA. Use Bethesda Units (BU) to report inhibitor levels.
6. Adverse Reactions/Side Effects
The most common adverse reactions (>0.5% of subjects) reported in clinical trials were Factor VIII inhibition in previously untreated patients (PUPs), dizziness and hypersensitivity.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
The safety, efficacy and pharmacokinetics of AFSTYLA have been evaluated in 258 previously treated patients (PTPs) and 24 previously untreated patients (PUPs) with severe hemophilia A (<1% endogenous Factor VIII activity) who received at least one dose of AFSTYLA as part of either routine prophylaxis, on-demand treatment of bleeding episodes or perioperative management in two completed clinical trials (an adult/adolescent study [≥12 to 65 years of age] and a pediatric study [<12 years of age]), and an extension study (0 to ≤65 years of age PTPs and 0 to ≤5 years old PUPs). Patients with a history of, or current FVIII inhibitors, or any first order family history of FVIII inhibitors, patients with known hypersensitivity (allergic reaction or anaphylaxis) to any FVIII product or hamster protein, and patients with evidence of thrombosis, including deep vein thrombosis, stroke, pulmonary embolism, myocardial infarction and arterial embolus within 3 months prior to Day 1 of the study were excluded from study participation.
One hundred eight (38.3%) subjects were children <12 years of age (59 [20.9%] 0 to <6 years and 49 [17.4%] ≥6 to <12 years), 14 (5.0%) were adolescents (≥12 to <18 years), and 160 (56.7%) were adults (≥18 to ≤65 years). In PTPs there have been a total of 28,418 exposure days (EDs), with at least 28,492 injections of AFSTYLA administered. In the completed studies, a total of 185 subjects achieved at least 50 EDs, of whom 60 subjects achieved ≥100 EDs. In PUPs there have been a total number of 5909 EDs, with 5914 injections, 21 subjects achieved ≥50 EDs.
Adverse reactions (ARs) (summarized in Table 3) were reported for 14 of 258 (5.4%) subjects in all studies. An adverse reaction of hypersensitivity resulted in the withdrawal of one subject. Among PTPs no subject developed neutralizing antibodies (inhibitors) to Factor VIII. No PTPs and PUPs developed antibodies to host cell proteins. No events of anaphylaxis or thrombosis were reported.
| MedDRA System Organ Class | Adverse Reactions | Number of Subjects n (%) |
|---|---|---|
|
||
| Immune system disorders | Hypersensitivity | 4 (1.6) |
| Nervous system disorders | Dizziness | 2 (0.8) |
| Paresthesia | 1 (0.4) | |
| Skin and subcutaneous tissue disorders | Rash | 1 (0.4) |
| Erythema | 1 (0.4) | |
| Pruritus | 1 (0.4) | |
| General disorders and administration site conditions | Pyrexia | 1 (0.4) |
| Injection-site pain | 1 (0.4) | |
| Chills | 1 (0.4) | |
| Feeling hot | 1 (0.4) | |
6.2 Immunogenicity
All subjects were monitored for inhibitory and binding antibodies to AFSTYLA, and binding antibodies to chinese hamster ovary (CHO) host cell proteins prior to the first infusion of AFSTYLA, at defined intervals during the studies and at the end of study visit.
Data from a completed clinical trial in previously untreated patients (PUPs) aged ≤5 years indicate that:
- 12 (50%; 95% CI: [29.1%, 70.9%]) of 24 treated subjects developed an inhibitor to Factor VIII.
- Of 22 subjects with at least 50 EDs or who developed inhibitor at any time, 6 (27.3%) developed high titer inhibitors (≥ 5BU) and 6 (27.3%) developed low titer inhibitors (<5BU).
The overall median EDs until initial inhibitor development was 10 EDs (range: 4 to 23). Of the 12 subjects who tested positive for inhibitors, 11 subjects have remained in the trial; 9 experienced successful eradication of the inhibitor. The median (range) EDs to inhibitor eradication was 37 (16 to 194). The median (range) time to inhibitor eradication was 14.3 (7.7 to 64.4) weeks. Two subjects with high titer inhibitors remain inhibitor positive: one subject was withdrawn from the immune tolerance induction (ITI) substudy after approximately 12 months, another subject completed ITI substudy after approximately 24 months.
No PTPs developed neutralizing antibodies (inhibitors) to Factor VIII or antibodies against CHO host cell proteins at any time during the completed clinical studies. Four subjects in the adult/adolescent study and 10 subjects in the pediatric study were negative for non-neutralizing anti-drug antibodies (ADAs) at screening and turned positive during the clinical study. Two of the adult/adolescent subjects and three of the pediatric subjects who developed ADAs were negative at end of study visit. No adverse events were associated with the development of ADAs. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, it may be misleading to compare the incidence of antibodies to AFSTYLA with the incidence of antibodies to other products.
6.3 Postmarketing Experience
The following adverse reaction has been identified during post-approval use of AFSTYLA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic systems disorders: Factor VIII inhibitor development
8. Use In Specific Populations
8.4 Pediatric Use
Safety and efficacy studies with AFSTYLA have been performed in 98 PTPs <18 years of age and in 24 PUPs ≤5 years. Fourteen adolescent subjects ≥12 to <18 years were enrolled in the adult/adolescent safety and efficacy study. Thirty-five subjects 0 to <6 years and 49 subjects ≥6 to <12 years were enrolled in a pediatric safety and efficacy study [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. Because clearance (based on per kg body weight) has been shown to be higher in the pediatric population 0 to <12 years, more frequent or higher doses of AFSTYLA based on body weight may be needed [see Clinical Pharmacology (12.3)].
11. Afstyla Description
AFSTYLA is a single-chain recombinant Factor VIII produced in chinese hamster ovary (CHO) cells. It is a construct where the B-domain occurring in wild type full-length Factor VIII has been truncated and 4 amino acids of the adjacent acidic a3 domain were removed (amino acids 765 to 1652 of full-length Factor VIII). AFSTYLA is expressed as a single-chain Factor VIII molecule with covalent linkage between heavy and light chains; thereby keeping the molecule in the single-chain form resulting in increased stability and increased von Willebrand Factor (VWF) affinity. Except for a new N-glycosylation site at the junction between heavy and light chains, the post-translational modifications are comparable to endogenous Factor VIII.
AFSTYLA is purified by a controlled multi-step process including two virus reduction steps complementing each other in their mode of action. No human or animal derived proteins are used in the purification or formulation processes.
AFSTYLA is a preservative-free, sterile, non-pyrogenic, lyophilized powder to be reconstituted with sterile water for injection (sWFI) for intravenous injection. AFSTYLA is available in single-dose vials containing the labeled amount of Factor VIII activity, expressed in IU. Each vial contains nominally 250, 500, 1000, 1500, 2000, 2500, or 3000 IU of AFSTYLA. The actual potency is labeled on each AFSTYLA vial and carton. After reconstitution of the lyophilized powder, all dosage strengths yield an almost colorless to slightly opalescent solution. The concentrations of excipients based on the vial size, as well as the amount of sWFI for reconstitution are provided in Table 4 below.
| Ingredient | 250 IU vial | 500 IU vial | 1000 IU vial | 1500 IU vial | 2000 IU vial | 2500 IU vial | 3000 IU vial |
|---|---|---|---|---|---|---|---|
| rVIII-Single Chain | 100 IU/mL | 200 IU/mL | 400 IU/mL | 300 IU/mL | 400 IU/mL | 500 IU/mL | 600 IU/mL |
| L-Histidine | 3.1 mg/mL | 3.1 mg/mL | 3.1 mg/mL | 3.1 mg/mL | 3.1 mg/mL | 3.1 mg/mL | 3.1 mg/mL |
| Polysorbate 80 | 0.2 mg/mL | 0.2 mg/mL | 0.2 mg/mL | 0.2 mg/mL | 0.2 mg/mL | 0.2 mg/mL | 0.2 mg/mL |
| Calcium chloride | 0.4 mg/mL | 0.4 mg/mL | 0.4 mg/mL | 0.4 mg/mL | 0.4 mg/mL | 0.4 mg/mL | 0.4 mg/mL |
| Sodium chloride | 16.4 mg/mL | 16.4 mg/mL | 16.4 mg/mL | 16.4 mg/mL | 16.4 mg/mL | 16.4 mg/mL | 16.4 mg/mL |
| Sucrose | 6 mg/mL | 6 mg/mL | 6 mg/mL | 6 mg/mL | 6 mg/mL | 6 mg/mL | 6 mg/mL |
| Water for Injection | 2.5 mL | 2.5 mL | 2.5 mL | 5 mL | 5 mL | 5 mL | 5 mL |
The number of units of Factor VIII administered is expressed in IU, which are related to the current WHO standard for Factor VIII products. One IU of Factor VIII activity in plasma is equivalent to that quantity of Factor VIII in 1 mL of normal plasma. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or in IU (relative to an International Standard for Factor VIII in plasma).
12. Afstyla - Clinical Pharmacology
12.1 Mechanism of Action
AFSTYLA is a recombinant protein that replaces the missing Coagulation Factor VIII needed for effective hemostasis. AFSTYLA is a single polypeptide chain with a truncated B-domain that allows for a covalent bridge to link the Factor VIII heavy and light chains. AFSTYLA has demonstrated a higher VWF affinity relative to full-length rFVIII.1 VWF stabilizes Factor VIII and protects it from degradation. Activated AFSTYLA has an amino acid sequence identical to endogenous FVIIIa.
12.2 Pharmacodynamics
Hemophilia A is an X-linked hereditary disorder of blood coagulation due to decreased levels of Factor VIII and results in bleeding into joints, muscles or internal organs, either spontaneously or as result of accidental or surgical trauma. Replacement therapy increases the plasma levels of Factor VIII enabling a temporary correction of the factor deficiency and correction of the bleeding tendencies.
14. Clinical Studies
The safety and efficacy of AFSTYLA were evaluated in two studies in PTPs: an open-label, multicenter, crossover safety, efficacy and pharmacokinetic study in adults/adolescents as well as in an open-label pharmacokinetic, efficacy and safety study in children. The safety and efficacy of AFSTYLA was also evaluated in previously untreated patients (PUPs) in an open-label, multicenter pharmacokinetic, efficacy and safety study. These studies characterized the PK of AFSTYLA and determined hemostatic efficacy in the control of bleeding events, the prevention of bleeding events in prophylaxis and in the adult/adolescent study determined hemostatic efficacy during perioperative management of bleeding in subjects undergoing surgical procedures.
The adult/adolescent study enrolled a total of 175 previously treated male subjects with severe hemophilia A (<1% endogenous Factor VIII activity). Subjects ranged in age from 12 to 65 years, including 14 adolescent subjects (≥12 to <18 years). Of the 175 enrolled subjects, 174 received at least one dose of AFSTYLA and 173 (99%) were evaluable for efficacy. A total of 161 subjects (92.5%) completed the study. A total of 120 (69.0%) subjects were treated for at least 50 EDs and 52 (29.9%) of those subjects were treated for at least 100 EDs. Subjects received a total of 14,592 injections with a median of 67.0 (range 1 to 395) injections per subject.
The pediatric study enrolled 84 previously treated male subjects with severe hemophilia A (35 subjects 0 to <6 years and 49 subjects ≥6 to <12 years). Of the 84 enrolled subjects, all received at least one dose of AFSTYLA and 83 (99%) were evaluable for efficacy. A total of 65 (77.4%) subjects were treated for at least 50 EDs and 8 (9.5%) of those subjects were treated for at least 100 EDs. Subjects received a total of 5,313 injections with a median of 59 (range 4 to 145) injections per subject.
Perioperative Management of Bleeding
Thirteen subjects in the adult/adolescent study underwent a total of 16 surgical procedures. Overall, investigators assessed hemostatic efficacy of AFSTYLA in perioperative management of bleeding as excellent in 15 of 16 surgeries and as good in 1 of 16 surgeries (see Table 9). Median factor consumption pre- and intra-operatively was 89.4 IU/kg (range 40.5 to 108.6 IU/kg).
Assessment of hemostasis during surgical procedures by the investigator was as follows:
Excellent: Hemostasis clinically not significantly different from normal (e.g., achieved hemostasis comparable to that expected during similar surgery in a non-factor deficient patient) in the absence of other hemostatic intervention and estimated blood loss during surgery is not more than 20% higher than the predicted blood loss for the intended surgery
Good: Normal or mildly abnormal hemostasis in terms of quantity and/or quality (e.g., slight oozing, prolonged time to hemostasis with somewhat increased bleeding compared to a non-factor deficient patient in the absence of other hemostatic intervention) or estimated blood loss is >20%, but ≤30% higher than the predicted blood loss for intended surgery
Moderate: Moderately abnormal hemostasis in terms of quantity and/or quality (e.g., moderate hemorrhage that is difficult to control) with estimated blood loss greater than what is defined as good
Poor/No Response: Severely abnormal hemostasis in terms of quantity and/or quality (e.g., severe hemorrhage that is difficult to control) and/or additional hemostatic intervention required with another FVIII product, cryoprecipitate, or plasma for complete resolution.
| Procedure | Efficacy Evaluation | Factor Consumption (IU/kg) (pre- and intra-operatively) |
|---|---|---|
| Extraction of wisdom teeth | Excellent | 51.09 |
| Abdominal hernia repair | Excellent | 47.89 |
| Elbow replacement | Excellent | 108.58 |
| Ankle arthroplasty | Excellent | 76.83 |
| Knee replacement (5) | Excellent (4), Good (1) | 92.49 100.9 67.26 105.79 86.09 |
| Cholecystectomy and Lengthening of the Achilles tendon combined with: Straightening of the right toes | Excellent Excellent | 105.95 |
| Circumcision (3) | Excellent (3) | 99.04 92.74 81.5 |
| Open reduction internal fixation (ORIF) right ankle | Excellent | 89.36 |
| Hardware removal, Right ankle | Excellent | 40.45 |
15. References
1. Zollner S, Raquet E, Claar Ph, Müller-Cohrs J, Metzner HJ, Weimer Th, Pragst I, Dickneite G, Schulte S. Non-clinical pharmacokinetics and pharmacodynamics of rVIII-SingleChain, a novel recombinant single-chain factor VIII, Thrombosis Research 2014; 134: 125-131.
16. How is Afstyla supplied
AFSTYLA is supplied in a kit containing a lyophilized powder in a single-dose vial labeled with the amount of Factor VIII activity, expressed in international units (IU). Actual Factor VIII activity in International Units (IU) is stated on the AFSTYLA carton and vial label.
AFSTYLA is packaged with Sterile Water for Injection, USP (2.5 mL for reconstitution of 250, 500 or 1000 IU or 5 mL for reconstitution of 1500, 2000, 2500, or 3000 IU AFSTYLA), one Mix2Vial filter transfer set, and one sterile alcohol swab. Components are not made of natural rubber latex.
| Nominal Strength | Fill Size Color Indicator | Kit NDC |
|---|---|---|
| 250 IU | Orange | 69911-474-02 |
| 500 IU | Blue | 69911-475-02 |
| 1000 IU | Green | 69911-476-02 |
| 1500 IU | Turquoise | 69911-480-02 |
| 2000 IU | Purple | 69911-477-02 |
| 2500 IU | Cool Grey | 69911-481-02 |
| 3000 IU | Yellow | 69911-478-02 |
17. Patient Counseling Information
Advise patients to:
- Read the FDA-approved Patient Labeling (Patient Product Information and Instructions for Use).
- Discontinue use of AFSTYLA in case of a hypersensitivity reaction and contact their healthcare provider and/or seek emergency care, depending on the severity of the reaction. Early signs of hypersensitivity reactions may include hives, itching, facial swelling, tightness of the chest, and wheezing [see Warnings and Precautions (5.1)].
- Contact their healthcare provider or hemophilia treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to Factor VIII replacement therapy, as in some cases this may be a manifestation of an inhibitor [see Warnings and Precautions (5.2)].
- Report any adverse reactions or problems following AFSTYLA administration to their healthcare provider.
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| AFSTYLA
ANTIHEMOPHILIC FACTOR (RECOMBINANT), SINGLE CHAIN
antihemophilic factor, human recombinant kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - CSL Behring Lengnau AG (480217014) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring GmbH | 326530474 | MANUFACTURE(69911-474, 69911-475, 69911-476, 69911-477, 69911-478, 69911-480, 69911-481) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring LLC | 058268293 | MANUFACTURE(69911-474, 69911-475, 69911-476, 69911-477, 69911-478, 69911-480, 69911-481) | |