Drug Detail:Aimovig (Erenumab)
Drug Class: CGRP inhibitors
Highlights of Prescribing Information
AIMOVIG® (erenumab-aooe) injection, for subcutaneous use
Initial U.S. Approval: 2018
Recent Major Changes
| Dosage and Administration (2.2) | 9/2022 |
Indications and Usage for Aimovig
AIMOVIG is a calcitonin gene-related peptide receptor antagonist indicated for the preventive treatment of migraine in adults. (1)
Aimovig Dosage and Administration
- For subcutaneous use only (2.1, 2.2)
- Recommended dosage is 70 mg once monthly; some patients may benefit from a dosage of 140 mg once monthly (2.1)
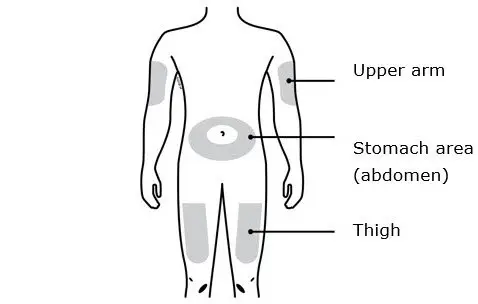
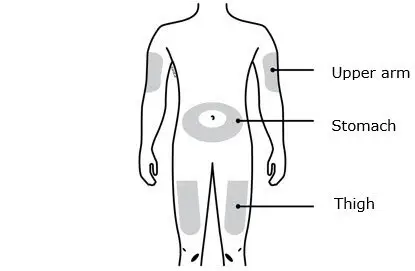
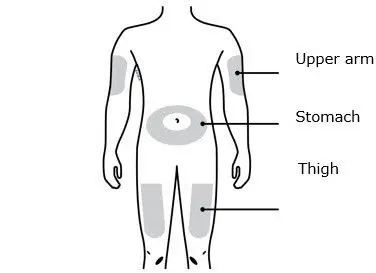
- Administer in the abdomen, thigh, or upper arm subcutaneously (2.2)
- See Dosage and Administration for important administration instructions (2.2)
Dosage Forms and Strengths
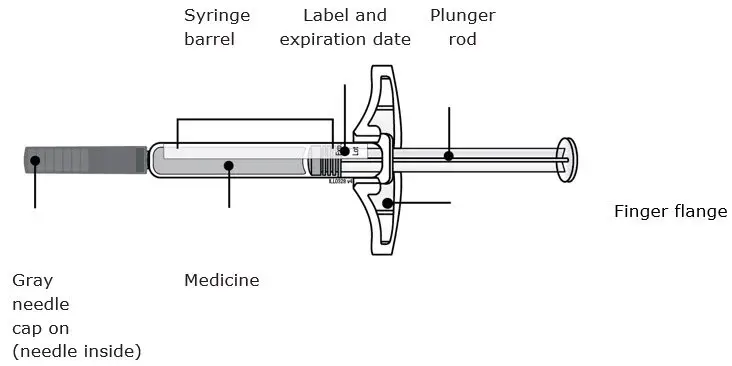
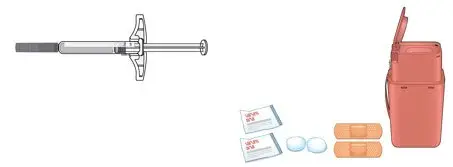
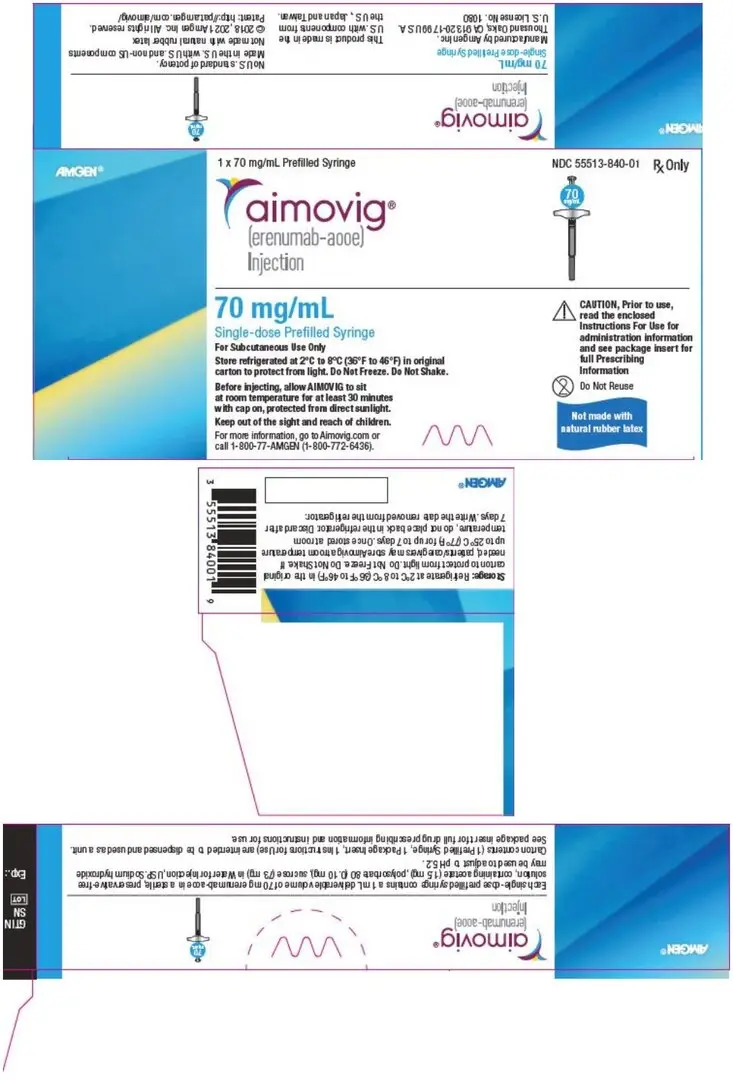
- Injection: 70 mg/mL solution in a single-dose prefilled SureClick® autoinjector (3)
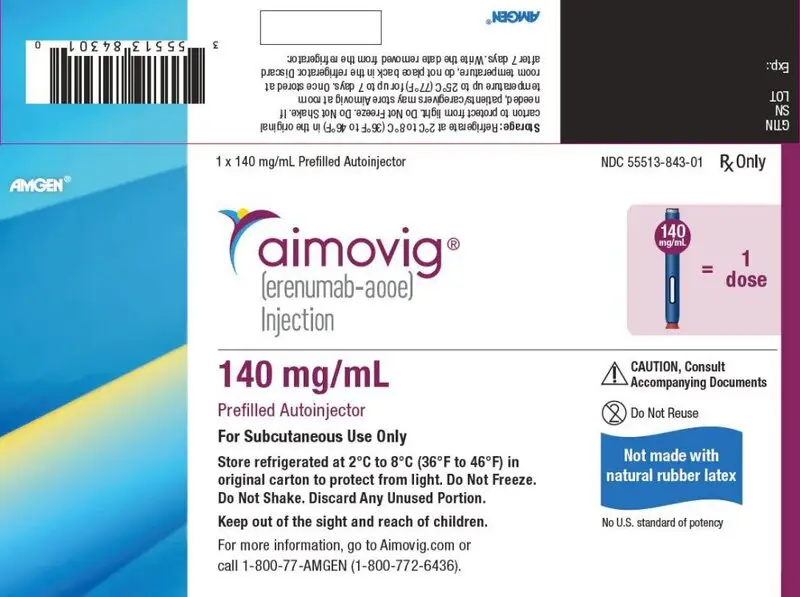
- Injection: 140 mg/mL solution in a single-dose prefilled SureClick® autoinjector (3)
- Injection: 70 mg/mL solution in a single-dose prefilled syringe (3)
- Injection: 140 mg/mL solution in a single-dose prefilled syringe (3)
Contraindications
AIMOVIG is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. (4)
Warnings and Precautions
- Hypersensitivity Reactions: If a serious hypersensitivity reaction occurs, discontinue administration of AIMOVIG and initiate appropriate therapy. Hypersensitivity reactions can occur within hours to more than one week after administration. (5.1)
- Constipation with Serious Complications: Serious complications of constipation may occur. (5.2)
- Hypertension: New-onset or worsening of pre-existing hypertension may occur. (5.3)
Adverse Reactions/Side Effects
The most common adverse reactions in AIMOVIG clinical studies (occurring in at least 3% of treated patients and more often than placebo) are injection site reactions and constipation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Medical Information at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2023
Related/similar drugs
Botox, propranolol, topiramate, Depakote, Topamax, divalproex sodium, valproic acidFull Prescribing Information
1. Indications and Usage for Aimovig
AIMOVIG is indicated for the preventive treatment of migraine in adults.
2. Aimovig Dosage and Administration
2.1 Recommended Dosing
The recommended dosage of AIMOVIG is 70 mg injected subcutaneously once monthly. Some patients may benefit from a dosage of 140 mg injected subcutaneously once monthly.
If a dose of AIMOVIG is missed, administer as soon as possible. Thereafter, AIMOVIG can be scheduled monthly from the date of the last dose.
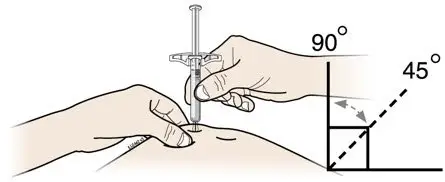
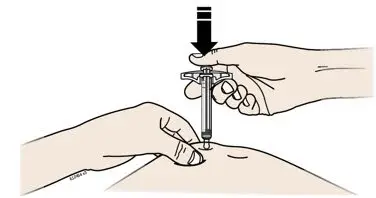
2.2 Important Administration Instructions
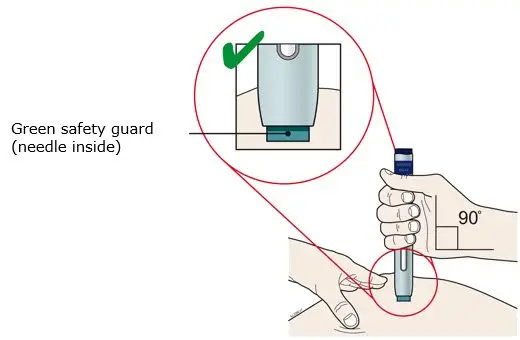
AIMOVIG is for subcutaneous use only.
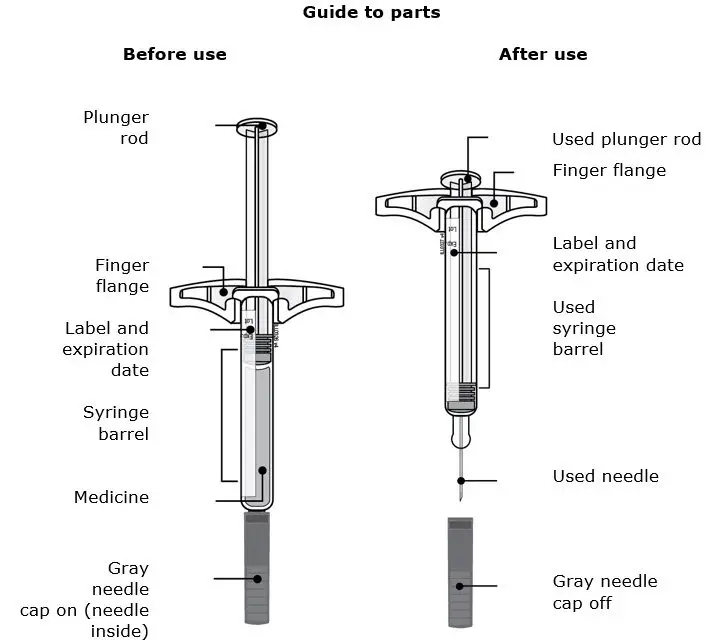
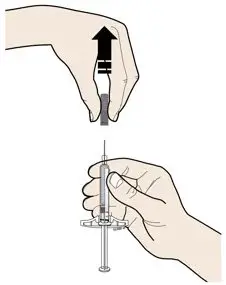
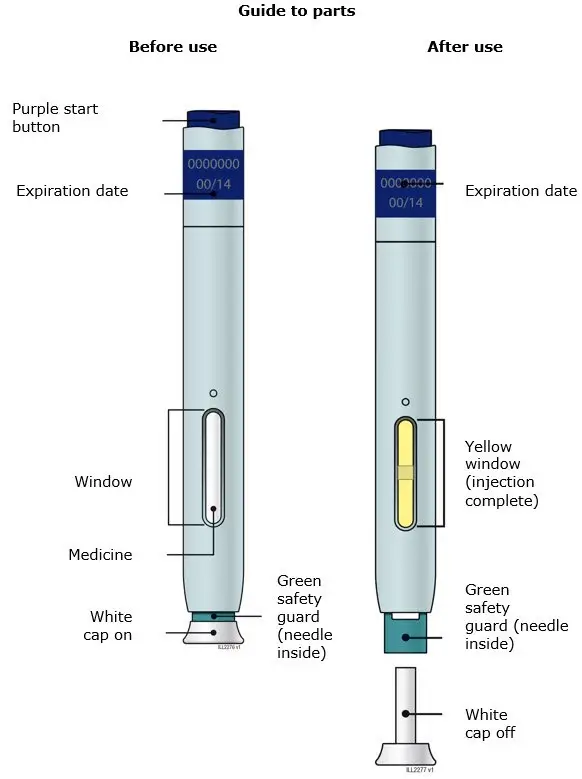
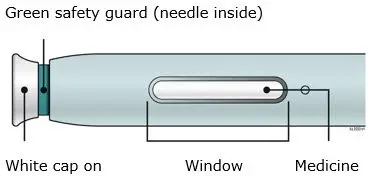
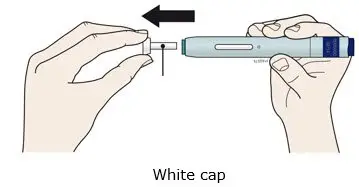
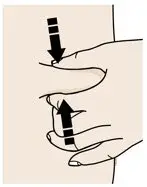
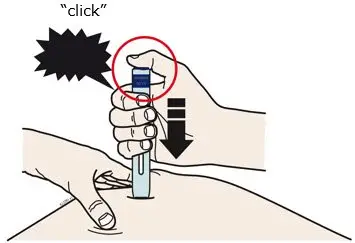
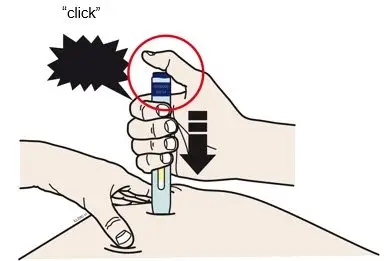
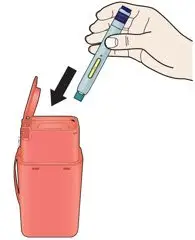
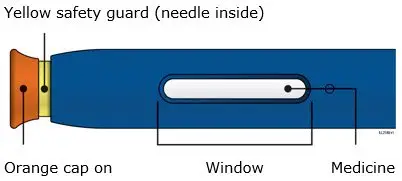
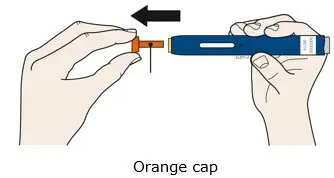
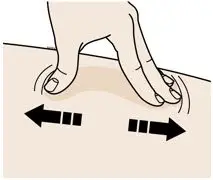
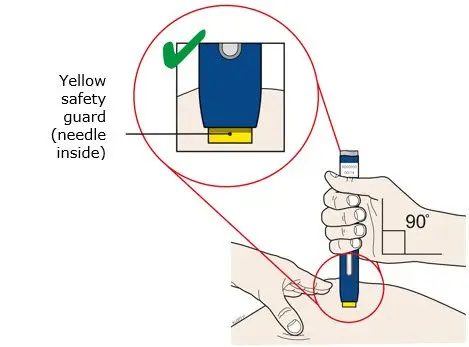
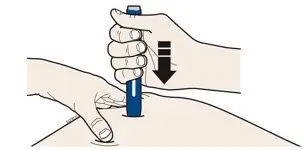
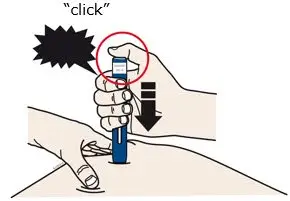
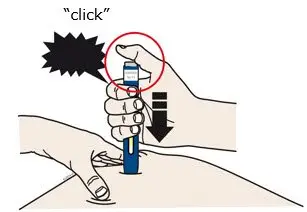
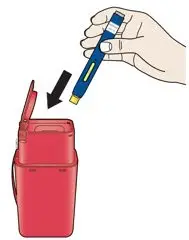
AIMOVIG is intended for patient self-administration. Prior to use, provide proper training to patients and/or caregivers on how to prepare and administer AIMOVIG using the single-dose prefilled autoinjector or single-dose prefilled syringe, including aseptic technique [see Instructions for Use]:
- Prior to subcutaneous administration, allow AIMOVIG to sit at room temperature for at least 30 minutes protected from direct sunlight [see How Supplied/Storage and Handling (16.2)]. This is important for administering the entire dose and helps minimize discomfort. Do not warm by using a heat source such as hot water or a microwave.
- Do not shake the product.
- Inspect visually for particulate matter and discoloration prior to administration [see Dosage Forms and Strengths (3)]. Do not use if the solution is cloudy or discolored or contains flakes or particles.
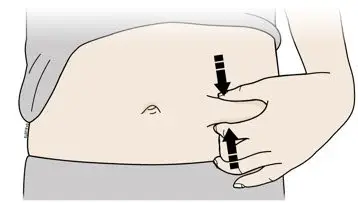
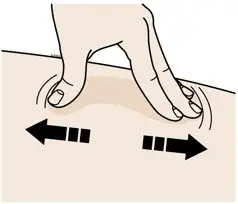
- Administer AIMOVIG in the abdomen, thigh, or upper arm subcutaneously. Do not inject into areas where the skin is tender, bruised, red, or hard.
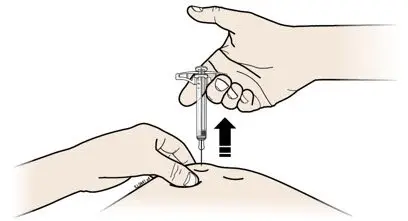
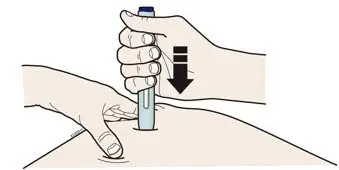
- Both prefilled autoinjector and prefilled syringe are single-dose and deliver the entire contents.
3. Dosage Forms and Strengths
AIMOVIG is a sterile, clear to opalescent, colorless to light yellow solution available as follows:
- Injection: 70 mg/mL in a single-dose prefilled SureClick® autoinjector
- Injection: 140 mg/mL in a single-dose prefilled SureClick® autoinjector
- Injection: 70 mg/mL in a single-dose prefilled syringe
- Injection: 140 mg/mL in a single-dose prefilled syringe
4. Contraindications
AIMOVIG is contraindicated in patients with serious hypersensitivity to erenumab-aooe or to any of the excipients. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, angioedema, and anaphylaxis, have been reported with AIMOVIG in postmarketing experience. Most hypersensitivity reactions were not serious and occurred within hours of administration, although some occurred more than one week after administration. If a serious or severe hypersensitivity reaction occurs, discontinue administration of AIMOVIG and initiate appropriate therapy [see Contraindications (4), and Patient Counseling Information (17)].
5.2 Constipation with Serious Complications
Constipation with serious complications has been reported following the use of AIMOVIG in the postmarketing setting. There were cases that required hospitalization, including cases where surgery was necessary. In a majority of these cases, the onset of constipation was reported after the first dose of AIMOVIG; however, patients have also presented with constipation later on in treatment. AIMOVIG was discontinued in most reported cases of constipation with serious complications. Constipation was one of the most common (up to 3%) adverse reactions reported in clinical studies [see Adverse Reactions (6.1)].
Monitor patients treated with AIMOVIG for severe constipation and manage as clinically appropriate [see Patient Counseling Information (17)]. The concurrent use of medications associated with decreased gastrointestinal motility may increase the risk for more severe constipation and the potential for constipation-related complications.
5.3 Hypertension
Development of hypertension and worsening of pre-existing hypertension have been reported following the use of AIMOVIG in the postmarketing setting. Many of the patients had pre-existing hypertension or risk factors for hypertension. There were cases requiring pharmacological treatment and, in some cases, hospitalization. Hypertension may occur at any time during treatment but was most frequently reported within seven days of dose administration. In the majority of the cases, the onset or worsening of hypertension was reported after the first dose. AIMOVIG was discontinued in many of the reported cases.
Monitor patients treated with AIMOVIG for new-onset hypertension, or worsening of pre-existing hypertension, and consider whether discontinuation of AIMOVIG is warranted if evaluation fails to establish an alternative etiology.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below and elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Constipation with Serious Complications [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of AIMOVIG has been evaluated in 2537 patients with migraine who received at least one dose of AIMOVIG, representing 3040.2 patient-years of exposure. Of these, 2271 patients were exposed to 70 mg or 140 mg once monthly for at least 6 months, 1305 patients were exposed for at least 12 months, and 216 patients were exposed through 5 years.
In placebo-controlled clinical studies (Studies 1, 2, and 3) of 2184 patients, 787 patients received at least one dose of AIMOVIG 70 mg once monthly, 507 patients received at least one dose of AIMOVIG 140 mg once monthly, and 890 patients received placebo during 3 months or 6 months of double-blind treatment [see Clinical Studies (14)]. Approximately 84% were female, 91% were white, and the mean age was 42 years at study entry.
The most common adverse reactions (incidence ≥ 3% and more often than placebo) in the migraine studies were injection site reactions and constipation. Table 1 summarizes the adverse reactions that occurred during the first 3 months in the migraine studies (Studies 1, 2, and 3).
| Adverse Reaction | AIMOVIG 70 mg Once Monthly N = 787 % | AIMOVIG 140 mg Once Monthly N = 507 % | Placebo N = 890 % |
|---|---|---|---|
|
|||
| Injection site reactions*,† | 6 | 5 | 3 |
| Constipation | 1 | 3 | 1 |
| Cramps, muscle spasms | < 1 | 2 | < 1 |
In Studies 1, 2, and 3, 1.3% of patients treated with AIMOVIG 70 mg or 140 mg discontinued double-blind treatment because of adverse events. The most frequent injection site reactions were injection site pain, injection site erythema, and injection site pruritus.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of AIMOVIG. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders: Hypersensitivity reactions, including rash, angioedema, and anaphylaxis [see Warnings and Precautions (5.1)].
Gastrointestinal Disorders: Constipation with serious complications [see Warnings and Precautions (5.2)], oral mucosal ulceration.
Skin and Subcutaneous Tissue Disorders: Rash, alopecia.
Vascular Disorders: Hypertension [see Warnings and Precautions (5.3)].
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of AIMOVIG did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
11. Aimovig Description
Erenumab-aooe is a human immunoglobulin G2 (IgG2) monoclonal antibody that has high affinity binding to the calcitonin gene-related peptide receptor. Erenumab-aooe is produced using recombinant DNA technology in Chinese hamster ovary (CHO) cells. It is composed of 2 heavy chains, each containing 456 amino acids, and 2 light chains of the lambda subclass, each containing 216 amino acids, with an approximate molecular weight of 150 kDa.
AIMOVIG (erenumab-aooe) injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to light yellow solution for subcutaneous administration. Each 1 mL 70 mg single-dose prefilled autoinjector and 70 mg single-dose prefilled glass syringe contains 70 mg erenumab-aooe, acetate (1.5 mg), polysorbate 80 (0.10 mg), and sucrose (73 mg). Each 1 mL 140 mg single-dose prefilled autoinjector and 140 mg single-dose prefilled glass syringe contains 140 mg erenumab-aooe, acetate (2.0 mg), polysorbate 80 (0.10 mg), and sucrose (65 mg). Enclosed within the autoinjector is a single-dose, prefilled glass syringe. The solution of AIMOVIG has a pH of 5.2.
12. Aimovig - Clinical Pharmacology
12.1 Mechanism of Action
Erenumab-aooe is a human monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) receptor and antagonizes CGRP receptor function.
12.2 Pharmacodynamics
In a randomized, double-blind, placebo-controlled study in healthy volunteers, concomitant administration of erenumab-aooe (140 mg intravenous, single-dose) with sumatriptan (12 mg subcutaneous, given as two 6 mg doses separated by one hour) had no effect on resting blood pressure compared with sumatriptan alone. AIMOVIG is for subcutaneous use only.
12.3 Pharmacokinetics
Erenumab-aooe exhibits non-linear kinetics as a result of binding to the CGRP receptor. The Cmax mean and AUClast mean following subcutaneous administration of a 70 mg once monthly and a 140 mg once monthly dose in healthy volunteers or migraine patients are included in Table 2.
Less than 2-fold accumulation was observed in trough serum concentrations (Cmin) for episodic and chronic migraine patients following subcutaneous administration of 70 mg once monthly and 140 mg once monthly doses (see Table 2). Serum trough concentrations approached steady state by 3 months of dosing. The effective half-life of erenumab-aooe is 28 days.
| AIMOVIG 70 mg Subcutaneously Once Monthly | AIMOVIG 140 mg Subcutaneously Once Monthly |
|
|---|---|---|
|
||
| Cmax mean (SD)*,† | 6.1 (2.1) mcg/mL | 15.8 (4.8) mcg/mL |
| AUClast mean (SD)*,† | 159 (58) day*mcg/mL | 505 (139) day*mcg/mL |
| Cmin (SD) | ||
| Episodic migraine | 5.7 (3.1) mcg/mL | 12.8 (6.5) mcg/mL |
| Chronic migraine | 6.2 (2.9) mcg/mL | 14.9 (6.5) mcg/mL |
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of erenumab-aooe.
The immunogenicity of AIMOVIG has been evaluated using an immunoassay for the detection of binding anti-erenumab-aooe antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
In controlled studies with AIMOVIG [see Clinical Studies (14)], the incidence of anti-erenumab-aooe antibody development was 6.2% (48/778) in patients receiving AIMOVIG 70 mg once monthly (2 of whom had in vitro neutralizing activity) and 2.6% (13/504) in patients receiving AIMOVIG 140 mg once monthly (none of whom had in vitro neutralizing activity). In a long-term study, including 12 weeks of double-blind treatment and up to 256 weeks of open-label treatment, the incidence of anti-erenumab-aooe antibody development was 11.1% (25/225) among patients whose AIMOVIG dose was 70 mg or 140 mg (2 of whom had in vitro neutralizing activity). The neutralizing anti-erenumab-aooe antibody positive rate may be underestimated because of limitations of the assay. Although these data do not demonstrate an impact of anti-erenumab-aooe antibody development on the efficacy or safety of AIMOVIG in these patients, the available data are too limited to make definitive conclusions.
14. Clinical Studies
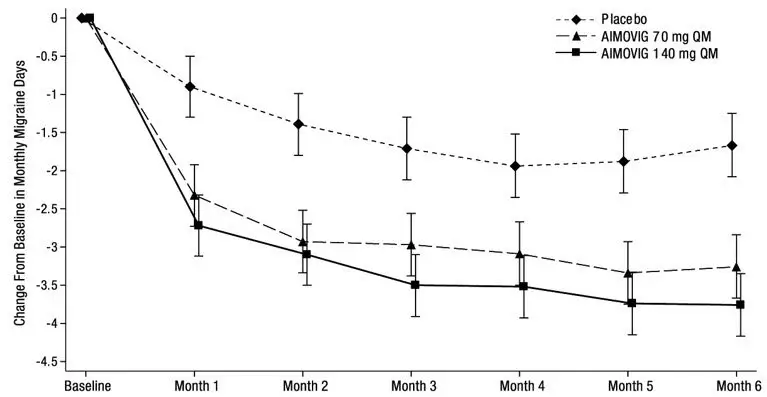
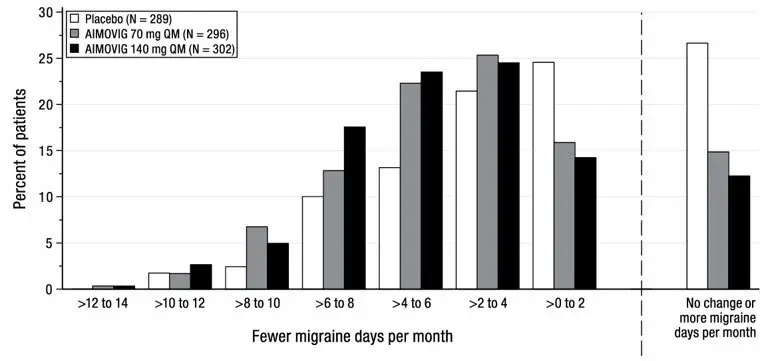
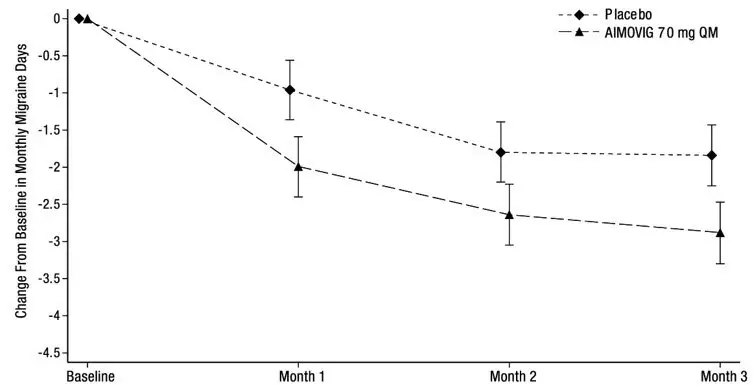
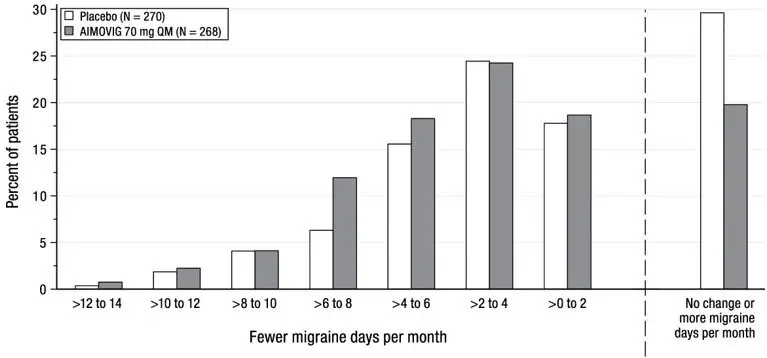
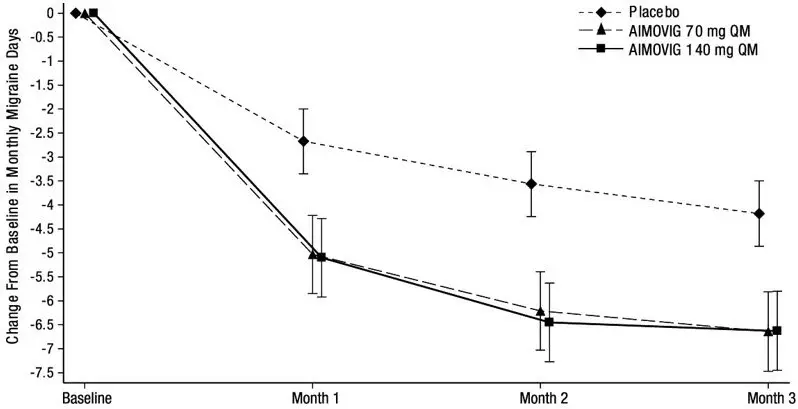
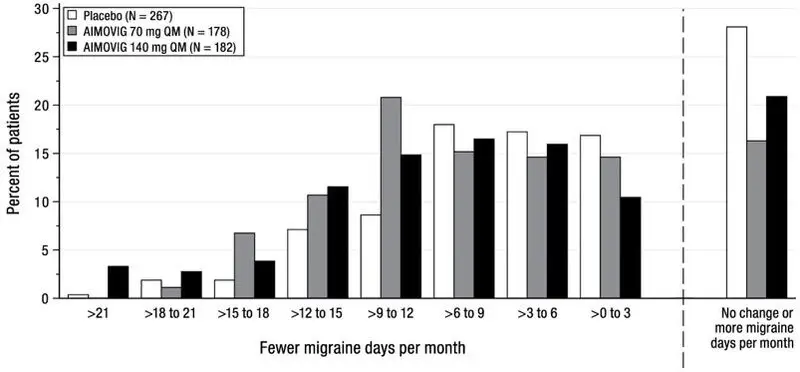
The efficacy of AIMOVIG was evaluated as a preventive treatment of episodic or chronic migraine in three randomized, double-blind, placebo-controlled studies: two studies in patients with episodic migraine (4 to 14 migraine days per month) (Study 1 and Study 2) and one study in patients with chronic migraine (≥ 15 headache days per month with ≥ 8 migraine days per month) (Study 3). The studies enrolled patients with a history of migraine, with or without aura, according to the International Classification of Headache Disorders (ICHD-III) diagnostic criteria.
16. How is Aimovig supplied
16.1 How Supplied
AIMOVIG (erenumab-aooe) injection is a sterile, clear to opalescent, colorless to light yellow solution for subcutaneous administration.
AIMOVIG prefilled autoinjectors and prefilled syringes are not made with natural rubber latex. Each single-dose prefilled SureClick® autoinjector or single-dose prefilled syringe of AIMOVIG contains a Type 1 glass syringe and stainless steel needle and delivers 1 mL of 70 mg/mL or 140 mg/mL solution.
AIMOVIG is supplied as follows:
SureClick® Autoinjector
- Pack of 1 autoinjector: 70 mg/mL single-dose prefilled autoinjector
NDC 55513-841-01 - Pack of 1 autoinjector: 140 mg/mL single-dose prefilled autoinjector
NDC 55513-843-01
Syringe
- Pack of 1 syringe: 70 mg/mL single-dose prefilled syringe
NDC 55513-840-01 - Pack of 1 syringe: 140 mg/mL single-dose prefilled syringe
NDC 55513-842-01
16.2 Storage and Handling
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until time of use.
- If removed from the refrigerator, AIMOVIG should be kept at room temperature (up to 25°C [77°F]) in the original carton and must be used within 7 days. Throw away AIMOVIG that has been left at room temperature for more than 7 days.
- Do not freeze.
- Do not shake.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
| AIMOVIG
erenumab-aooe injection |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AIMOVIG
erenumab-aooe injection |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| AIMOVIG
erenumab-aooe injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AIMOVIG
erenumab-aooe injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amgen Inc (039976196) |









 This printed material is recyclable.
This printed material is recyclable.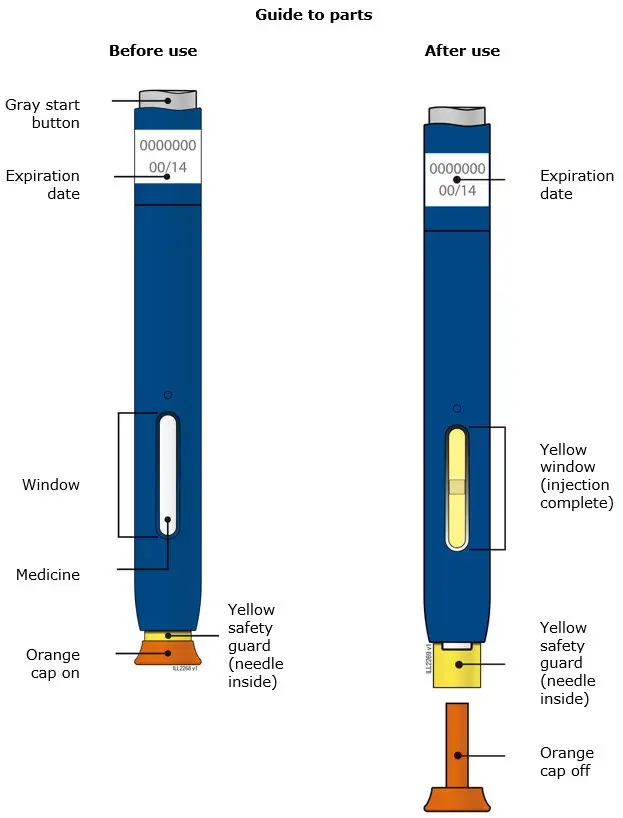




 This printed material is recyclable.
This printed material is recyclable.