Drug Detail:Akeega (Niraparib and abiraterone acetate)
Drug Class:
Highlights of Prescribing Information
AKEEGA™ (niraparib and abiraterone acetate) tablets, for oral use
Initial U.S. Approval: 2023
Indications and Usage for Akeega
AKEEGA is a combination of niraparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, and abiraterone acetate, a CYP17 inhibitor indicated with prednisone for the treatment of adult patients with deleterious or suspected deleterious BRCA-mutated ( BRCAm) metastatic castration-resistant prostate cancer (mCRPC). Select patients for therapy based on an FDA-approved test for AKEEGA. ( 1, 2.1)
Akeega Dosage and Administration
The recommended dosage of AKEEGA is 200 mg niraparib/1,000 mg abiraterone acetate orally once daily in combination with 10 mg prednisone daily until disease progression or unacceptable toxicity. ( 2.2)
Patients receiving AKEEGA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. ( 2.2)
Take AKEEGA on an empty stomach. Do not eat food two hours before and one hour after taking AKEEGA. ( 2.2)
For adverse reactions, consider interruption of treatment, dose reduction, or dose discontinuation. ( 2.3)
Dosage Forms and Strengths
Tablets:
- 50 mg niraparib/500 mg abiraterone acetate ( 3)
- 100 mg niraparib/500 mg abiraterone acetate ( 3)
Contraindications
None. ( 4)
Warnings and Precautions
- Myelodysplastic Syndrome/Acute Myeloid Leukemia (MDS/AML): MDS/AML, including cases with fatal outcome, has been observed in patients treated with niraparib, a component of AKEEGA. Monitor patients for hematological toxicity and discontinue if MDS/AML is confirmed. ( 5.1)
- Myelosuppression: Test complete blood counts weekly for the first month, every two weeks for the next two months, monthly for the remainder of the first year, then every other month, and as clinically indicated. ( 2.3, 5.2)
- Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions: Monitor patients for hypertension, hypokalemia, and fluid retention at least weekly for the first two months, then once a month. Closely monitor patients whose underlying medical conditions might be compromised by increases in blood pressure, hypokalemia, or fluid retention. Control hypertension and correct hypokalemia before and during treatment with AKEEGA. ( 5.3)
- Hepatotoxicity: Can be severe and fatal. Monitor liver function and modify, interrupt, or discontinue treatment as recommended. ( 2.3, 5.4)
- Adrenocortical insufficiency: Monitor for symptoms and signs of adrenocortical insufficiency. Increased dosage of corticosteroids may be indicated before, during and after stressful situations. ( 5.5)
- Hypoglycemia: Severe hypoglycemia has been reported when abiraterone acetate, a component of AKEEGA, was administered to patients receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide. Monitor blood glucose in patients with diabetes during and assess if antidiabetic agent dose modifications are required. ( 5.6)
- Increased fractures and mortality in combination with radium Ra 223 dichloride: Use of AKEEGA plus prednisone in combination with radium Ra 223 dichloride is not recommended. ( 5.7)
- Posterior Reversible Encephalopathy Syndrome (PRES): PRES has been observed in patients treated with niraparib, a component of AKEEGA. Discontinue AKEEGA if PRES is confirmed. ( 5.8)
- Embryo-Fetal Toxicity: AKEEGA can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception. ( 5.9, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common adverse reactions (≥10%), including laboratory abnormalities, are decreased hemoglobin, decreased lymphocytes, decreased white blood cells, musculoskeletal pain, fatigue, decreased platelets, increased alkaline phosphatase, constipation, hypertension, nausea, decreased neutrophils, increased creatinine, increased potassium, decreased potassium, increased AST, increased ALT, edema, dyspnea, decreased appetite, vomiting, dizziness, COVID-19, headache, abdominal pain, hemorrhage, urinary tract infection, cough, insomnia, increased bilirubin, weight decreased, arrhythmia, fall, and pyrexia. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-526-7736 (1-800-JANSSEN) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP3A4 Inducers: Avoid coadministration. ( 7.1)
- CYP2D6 Substrates: Avoid coadministration of AKEEGA with CYP2D6 substrates for which minimal changes in concentration may lead to serious toxicities. If alternative treatments cannot be used, consider a dose reduction of the concomitant CYP2D6 substrate. ( 7.2)
Use In Specific Populations
- Moderate or Severe Hepatic impairment: Avoid use. ( 8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
Full Prescribing Information
1. Indications and Usage for Akeega
AKEEGA with prednisone is indicated for the treatment of adult patients with deleterious or suspected deleterious BRCA-mutated ( BRCAm) metastatic castration-resistant prostate cancer (mCRPC). Select patients for therapy based on an FDA-approved test for AKEEGA [see Dosage and Administration (2.1)] .
2. Akeega Dosage and Administration
2.1 Patient Selection
Select patients for the treatment of mCRPC with AKEEGA based on the presence of a BRCAgene alteration [see Clinical Studies (14)] .
Information on FDA-approved tests is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosage of AKEEGA is 200 mg niraparib/1,000 mg abiraterone acetate orally once daily in combination with 10 mg prednisone daily until disease progression or unacceptable toxicity.
Patients receiving AKEEGA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. Take AKEEGA on an empty stomach. Do not eat food two hours before and one hour after taking AKEEGA. Swallow tablets whole with water. Do not break, crush, or chew tablets.
If a patient misses a dose, instruct patients to take the dose as soon as possible on the same day and resume their next dose at the normal schedule the following day.
2.3 Dosage Modification for Adverse Reactions
The recommended dosage modifications for AKEEGA are provided in Table 1.
Treatment with AKEEGA should not be reinitiated until the toxicity has resolved to Grade 1 or baseline. If the toxicity is attributed to one component of AKEEGA, the other component of AKEEGA may be continued as a single agent at the current dose until the adverse reaction resolves and AKEEGA can be resumed (see Table 1).
| Adverse Reaction | Severity | Dosage Modification |
|---|---|---|
|
||
| Myelosuppression
[see Warnings and Precautions (5.2)] | Hemoglobin <8 g/dL |
|
| Platelet count <100,000/mcL | First occurrence:
|
|
| Neutrophil <1,000/mcL |
|
|
| Hematologic adverse reaction requiring transfusion |
|
|
| Hepatotoxicity
[see Warnings and Precautions (5.4)] | ALT and/or AST greater than 5 × ULN or total bilirubin greater than 3 × ULN |
|
| Other non-hematological adverse reactions that persist despite medical management [see Warnings and Precautions (5)and Adverse Reactions (6.1)] | Grade 3 or 4 † |
|
3. Dosage Forms and Strengths
Tablets
- 50 mg niraparib/500 mg abiraterone acetate: yellowish orange to yellowish brown, oval, film-coated tablets debossed with "N 50 A" on one side and plain on the other side.
- 100 mg niraparib/500 mg abiraterone acetate: orange, oval, film-coated tablets debossed with "N 100 A" on one side and plain on the other side.
5. Warnings and Precautions
5.1 Myelodysplastic Syndrome/Acute Myeloid Leukemia
AKEEGA may cause myelodysplastic syndrome/acute myeloid leukemia (MDS/AML).
MDS/AML, including cases with fatal outcome, has been observed in patients treated with niraparib, a component of AKEEGA.
All patients treated with niraparib who developed secondary MDS/cancer-therapy-related AML had received previous chemotherapy with platinum agents and/or other DNA-damaging agents, including radiotherapy.
For suspected MDS/AML or prolonged hematological toxicities, refer the patient to a hematologist for further evaluation. Discontinue AKEEGA if MDS/AML is confirmed.
5.2 Myelosuppression
AKEEGA may cause myelosuppression (anemia, thrombocytopenia, or neutropenia).
In MAGNITUDE Cohort 1, Grade 3–4 anemia, thrombocytopenia, and neutropenia were reported, respectively in 28%, 8%, and 7% of patients receiving AKEEGA. Overall, 27% of patients required a red blood cell transfusion, including 11% who required multiple transfusions. Discontinuation due to anemia occurred in 3% of patients.
Monitor complete blood counts weekly during the first month of AKEEGA treatment, every two weeks for the next two months, monthly for the remainder of the first year and then every other month, and as clinically indicated. Do not start AKEEGA until patients have adequately recovered from hematologic toxicity caused by previous therapy. If hematologic toxicities do not resolve within 28 days following interruption, discontinue AKEEGA and refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics [see Dosage and Administration (2.3)] .
5.3 Hypokalemia, Fluid Retention, and Cardiovascular Adverse Reactions
AKEEGA may cause hypokalemia and fluid retention as a consequence of increased mineralocorticoid levels resulting from CYP17 inhibition [see Clinical Pharmacology (12.1)] . In post-marketing experience, QT prolongation and Torsades de Pointes have been observed in patients who develop hypokalemia while taking abiraterone acetate, a component of AKEEGA. Hypertension and hypertensive crisis have also been reported in patients treated with niraparib, a component of AKEEGA.
In MAGNITUDE Cohort 1, which used prednisone 10 mg daily in combination with AKEEGA, Grades 3–4 hypokalemia was detected in 2.7% of patients on the AKEEGA arm and Grades 3–4 hypertension were observed in 14% of patients on the AKEEGA arm.
The safety of AKEEGA in patients with New York Heart Association (NYHA) Class II to IV heart failure has not been established because these patients were excluded from MAGNITUDE.
Monitor patients for hypertension, hypokalemia, and fluid retention at least weekly for the first two months, then once a month. Closely monitor patients whose underlying medical conditions might be compromised by increases in blood pressure, hypokalemia, or fluid retention, such as those with heart failure, recent myocardial infarction, cardiovascular disease, or ventricular arrhythmia. Control hypertension and correct hypokalemia before and during treatment with AKEEGA.
Discontinue AKEEGA in patients who develop hypertensive crisis or other severe cardiovascular adverse reactions.
5.4 Hepatotoxicity
AKEEGA may cause hepatotoxicity.
Hepatotoxicity in patients receiving abiraterone acetate, a component of AKEEGA, has been reported in clinical trials. In post-marketing experience, there have been abiraterone acetate-associated severe hepatic toxicity, including fulminant hepatitis, acute liver failure, and deaths.
In MAGNITUDE Cohort 1, Grade 3–4 ALT or AST increases (at least 5 × ULN) were reported in 1.8% of patients. The safety of AKEEGA in patients with moderate or severe hepatic impairment has not been established as these patients were excluded from MAGNITUDE.
Measure serum transaminases (ALT and AST) and bilirubin levels prior to starting treatment with AKEEGA, every two weeks for the first three months of treatment and monthly thereafter. Promptly measure serum total bilirubin, AST, and ALT if clinical symptoms or signs suggestive of hepatotoxicity develop. Elevations of AST, ALT, or bilirubin from the patient's baseline should prompt more frequent monitoring and may require dosage modifications [see Dosage and Administration (2.3)].
Permanently discontinue AKEEGA for patients who develop a concurrent elevation of ALT greater than 3 × ULN and total bilirubin greater than 2 × ULN in the absence of biliary obstruction or other causes responsible for the concurrent elevation, or in patients who develop ALT or AST ≥20 × ULN at any time after receiving AKEEGA.
5.5 Adrenocortical Insufficiency
AKEEGA may cause adrenal insufficiency.
Adrenocortical insufficiency has been reported in clinical trials in patients receiving abiraterone acetate, a component of AKEEGA, in combination with prednisone, following interruption of daily steroids and/or with concurrent infection or stress. Monitor patients for symptoms and signs of adrenocortical insufficiency, particularly if patients are withdrawn from prednisone, have prednisone dose reductions, or experience unusual stress. Symptoms and signs of adrenocortical insufficiency may be masked by adverse reactions associated with mineralocorticoid excess seen in patients treated with abiraterone acetate. If clinically indicated, perform appropriate tests to confirm the diagnosis of adrenocortical insufficiency. Increased doses of corticosteroids may be indicated before, during, and after stressful situations.
5.6 Hypoglycemia
AKEEGA may cause hypoglycemia in patients being treated with other medications for diabetes.
Severe hypoglycemia has been reported when abiraterone acetate, a component of AKEEGA, was administered to patients receiving medications containing thiazolidinediones (including pioglitazone) or repaglinide [see Drug Interactions (7.2)] .
Monitor blood glucose in patients with diabetes during and after discontinuation of treatment with AKEEGA. Assess if antidiabetic drug dosage needs to be adjusted to minimize the risk of hypoglycemia.
5.7 Increased Fractures and Mortality in Combination with Radium 223 Dichloride
AKEEGA with prednisone is not recommended for use in combination with Ra-223 dichloride outside of clinical trials.
The clinical efficacy and safety of concurrent initiation of abiraterone acetate plus prednisone/prednisolone and radium Ra 223 dichloride was assessed in a randomized, placebo-controlled multicenter study (ERA-223 trial) in 806 patients with asymptomatic or mildly symptomatic castration-resistant prostate cancer with bone metastases. The study was unblinded early based on an Independent Data Monitoring Committee recommendation.
At the primary analysis, increased incidences of fractures (29% vs 11%) and deaths (39% vs 36%) have been observed in patients who received abiraterone acetate plus prednisone/prednisolone in combination with radium Ra 223 dichloride compared to patients who received placebo in combination with abiraterone acetate plus prednisone.
It is recommended that subsequent treatment with Ra-223 not be initiated for at least five days after the last administration of AKEEGA, in combination with prednisone.
5.8 Posterior Reversible Encephalopathy Syndrome
AKEEGA may cause Posterior Reversible Encephalopathy Syndrome (PRES).
PRES has been observed in patients treated with niraparib as a single agent at higher than the recommended dose of niraparib included in AKEEGA.
Monitor all patients treated with AKEEGA for signs and symptoms of PRES. If PRES is suspected, promptly discontinue AKEEGA and administer appropriate treatment. The safety of reinitiating AKEEGA in patients previously experiencing PRES is not known.
5.9 Embryo-Fetal Toxicity
The safety and efficacy of AKEEGA have not been established in females. Based on animal reproductive studies and mechanism of action, AKEEGA can cause fetal harm and loss of pregnancy when administered to a pregnant female [see Clinical Pharmacology (12.1)] .
Niraparib has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see Warnings and Precautions (5.2)and Nonclinical Toxicology (13.1)] .
In animal reproduction studies, oral administration of abiraterone acetate to pregnant rats during organogenesis caused adverse developmental effects at maternal exposures approximately ≥ 0.03 times the human exposure (AUC) at the recommended dose.
Advise males with female partners of reproductive potential to use effective contraception during treatment and for 4 months after the last dose of AKEEGA [see Use in Specific Populations (8.1, 8.3)] . Females who are or may become pregnant should handle AKEEGA with protection, e.g., gloves [see How Supplied/Storage and Handling (16)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed elsewhere in the labeling:
- Myelodysplastic syndrome/acute myeloid leukemia [see Warnings and Precautions (5.1)]
- Myelosuppression [see Warnings and Precautions (5.2)]
- Hypokalemia, fluid retention, and cardiovascular adverse reactions [see Warnings and Precautions (5.3)]
- Hepatotoxicity [see Warnings and Precautions (5.4)]
- Adrenocortical insufficiency [see Warnings and Precautions (5.5)]
- Hypoglycemia [see Warnings and Precautions (5.6)]
- Increased fractures and mortality in combination with Radium 223 Dichloride [see Warnings and Precautions (5.7)]
- Posterior reversible encephalopathy syndrome [see Warnings and Precautions (5.8)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety population described in the WARNINGS and PRECAUTIONS reflect exposure to AKEEGA (niraparib 200 mg and abiraterone acetate 1,000 mg) in combination with prednisone 10 mg once daily in BRCA-mutated patients in Cohort 1 (N=113) of MAGNITUDE.
BRCA-mutated Metastatic Castration-Resistant Prostate Cancer
The safety of AKEEGA in patients with BRCAm mCRPC was evaluated in Cohort 1 of MAGNITUDE [see Clinical Studies (14.1)]. Patients were randomized to receive either AKEEGA (niraparib 200 mg and abiraterone acetate 1,000 mg once daily) (n=113), or placebo and abiraterone acetate (n=112) until unacceptable toxicity or progression. Patients in both arms also received prednisone 10 mg daily. The median duration of exposure for AKEEGA was 18 months (range: 0 to 37 months).
Serious adverse reactions occurred in 41% of patients who received AKEEGA. Serious adverse reactions reported in >2% of patients included COVID-19 (7%), anemia (4.4%), pneumonia (3.5%), and hemorrhage (3.5%). Fatal adverse reactions occurred in 9% of patients who received AKEEGA, including COVID-19 (5%), cardiopulmonary arrest (1%), dyspnea (1%), pneumonia (1%), and septic shock (1%).
Permanent discontinuation of any component of AKEEGA due to an adverse reaction occurred in 15% of patients. Adverse reactions which resulted in permanent discontinuation of AKEEGA in > 2% of patients included COVID-19 (4.4%), anemia (2.7%), asthenia (2.7%), and vomiting (2.7%).
Dosage interruptions of any component of AKEEGA due to an adverse reaction occurred in 50% of patients. Adverse reactions which required dosage interruption in > 2% of patients included anemia (23%), thrombocytopenia (12%), neutropenia (7%), COVID-19 (6%), fatigue (3.5%), asthenia (3.5%), nausea (3.5%), pneumonia (2.7%), hematuria (2.7%), and vomiting (2.7%).
Dose reductions of any component of AKEEGA due to an adverse reaction occurred in 28% of patients. Adverse reactions which required dose reductions in > 2% of patients included anemia (12%), thrombocytopenia (4.4%), and fatigue (2.7%).
The most common adverse reactions (>10%) in patients who received AKEEGA were musculoskeletal pain, fatigue, constipation, hypertension, nausea, edema, dyspnea, decreased appetite, vomiting, dizziness, COVID-19, headache, abdominal pain, hemorrhage, urinary tract infection, cough, insomnia, weight decreased, arrhythmia, fall, and pyrexia.
The most common select laboratory abnormalities (>10%) that worsened from baseline in patients who received AKEEGA were hemoglobin decreased, platelets decreased, neutrophils decreased, creatinine increased, potassium decreased, AST increased, ALT increased, and bilirubin increased.
Tables 2 and 3 summarize adverse reactions and laboratory abnormalities for patients with BRCAm mCRPC in MAGNITUDE, respectively.
| AKEEGA with Prednisone
N=113 | Placebo with Abiraterone Acetate and Prednisone
N=112 |
|||
|---|---|---|---|---|
| Adverse Reaction | All Grades
% | Grade 3 or 4
% | All Grades
% | Grade 3 or 4
% |
|
||||
| Musculoskeletal pain * | 44 | 4 | 42 | 5 |
| Fatigue * | 43 | 5 | 30 | 4 |
| Constipation | 34 | 1 | 20 | 0 |
| Hypertension * | 33 | 14 | 27 | 17 |
| Nausea | 33 | 1 | 21 | 0 |
| Edema * | 17 | 0 | 9 | 0 |
| Dyspnea * | 15 | 1 | 8 | 3 |
| Decreased appetite | 15 | 2 | 8 | 0 |
| Vomiting | 15 | 0 | 7 | 1 |
| Dizziness * | 14 | 0 | 10 | 0 |
| COVID-19 * | 13 | 7 | 9 | 4 |
| Abdominal pain * | 12 | 2 | 12 | 1 |
| Hemorrhage * | 12 | 2 | 8 | 1 |
| Headache | 12 | 1 | 9 | 0 |
| Urinary tract infection * | 12 | 3 | 9 | 1 |
| Cough * | 12 | 0 | 6 | 0 |
| Insomnia | 12 | 0 | 4 | 0 |
| Weight decreased | 10 | 1 | 4 | 1 |
| Arrhythmia * | 10 | 2 | 4 | 1 |
| Fall | 10 | 1 | 13 | 4 |
| Pyrexia * | 10 | 2 | 6 | 0 |
Clinically relevant adverse events that occurred in <10% of patients receiving niraparib and abiraterone acetate plus prednisone were rash (7%), ALT increased (5%), AST increased (5%), cerebrovascular accident (4.4%), pulmonary embolism (2.7%), deep vein thrombosis (2.7%), and acute kidney injury (2.7%).
| AKEEGA with Prednisone
*
N=113 | Placebo with Abiraterone Acetate and Prednisone
*
N=112 |
|||
|---|---|---|---|---|
| Laboratory Abnormality | All Grades
(%) | Grade 3 or 4
(%) | All Grades
(%) | Grade 3 or 4
(%) |
|
||||
| Hematology | ||||
| Hemoglobin decreased | 67 | 26 | 53 | 7 |
| Lymphocyte decreased | 55 | 22 | 32 | 13 |
| WBC decreased | 48 | 6 | 18 | 0.9 |
| Platelets decreased | 37 | 8 | 22 | 1.8 |
| Neutrophils decreased | 32 | 7 | 16 | 2.7 |
| Chemistry | ||||
| ALP increased | 34 | 1.8 | 29 | 1.8 |
| Creatinine increased | 30 | 0 | 13 | 1.8 |
| Potassium increased | 25 | 0.9 | 21 | 3.6 |
| Potassium decreased | 20 | 5 | 20 | 5 |
| AST increased | 20 | 1.8 | 25 | 2.7 |
| ALT increased | 18 | 0.9 | 17 | 4.5 |
| Bilirubin increased | 12 | 0 | 10 | 0.9 |
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of AKEEGA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 113 patients with BRCAgene alteration(s) who received AKEEGA in MAGNITUDE, 34.5% of patients were less than 65 years, 38.9% of patients were 65 years to 74 years, and 26.5% were 75 years and over.
There was an insufficient number of patients with BRCAgene alteration(s) treated with AKEEGA in MAGNITUDE to accurately characterize efficacy or safety by age.
10. Overdosage
In the event of an overdose, administration of AKEEGA should be stopped and general supportive measures undertaken, including monitoring for arrhythmias and cardiac failure and assessing liver function.
There is no specific treatment in the event of AKEEGA overdose.
11. Akeega Description
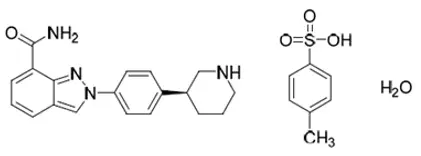
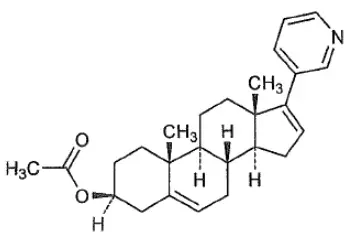
AKEEGA™ (niraparib and abiraterone acetate) tablets contain niraparib tosylate (as the monohydrate) and abiraterone acetate.
12. Akeega - Clinical Pharmacology
12.1 Mechanism of Action
Niraparib is an inhibitor of PARP enzymes, including PARP-1 and PARP-2, that play a role in DNA repair. In vitrostudies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis, and cell death. Increased niraparib‑induced cytotoxicity was observed in tumor cell lines with or without deficiencies in BRCA1/2. Niraparib decreased tumor growth in mouse xenograft models of human cancer cell lines with deficiencies in BRCA1/2and in human patient‑derived xenograft tumor models with homologous recombination deficiency (HRD) that had either mutated or wild-type BRCA1/2.
Abiraterone acetate is converted in vivoto abiraterone, an androgen biosynthesis inhibitor, that inhibits 17 α-hydroxylase/C17,20-lyase (CYP17). This enzyme is expressed in testicular, adrenal, and prostatic tumor tissues and is required for androgen biosynthesis.
CYP17 catalyzes two sequential reactions: 1) the conversion of pregnenolone and progesterone to their 17α-hydroxy derivatives by 17α-hydroxylase activity and 2) the subsequent formation of dehydroepiandrosterone (DHEA) and androstenedione, respectively, by C17, 20 lyase activity. DHEA and androstenedione are androgens and are precursors of testosterone. Inhibition of CYP17 by abiraterone can also result in increased mineralocorticoid production by the adrenals [see Warnings and Precautions (5.9)] .
Androgen sensitive prostatic carcinoma responds to treatment that decreases androgen levels. Androgen deprivation therapies, such as treatment with GnRH agonists or orchiectomy, decrease androgen production in the testes but do not affect androgen production by the adrenals or in the tumor.
Abiraterone decreased serum testosterone and other androgens in patients in the placebo-controlled clinical trial. It is not necessary to monitor the effect of abiraterone on serum testosterone levels.
Changes in serum prostate specific antigen (PSA) levels may be observed but have not been shown to correlate with clinical benefit in individual patients.
In mouse xenograft models of prostate cancer, the combination of niraparib and abiraterone acetate increased anti-tumor activity when compared to either drug alone.
12.2 Pharmacodynamics
The exposure-response relationship and time-course of pharmacodynamic response for the safety and effectiveness of AKEEGA have not been fully characterized.
12.3 Pharmacokinetics
Specific Populations
No clinically significant effects on the PK of niraparib and abiraterone were observed based on body weight (43.3–165 kg for niraparib and 46–165 kg for abiraterone), age (45–90 years for niraparib and 43–90 years for abiraterone), race/ethnicity (White, Asian, and Hispanic) and mild to moderate renal impairment (CLcr: 30–90 mL/min). Severe renal impairment (CLcr: 15–30 mL/min) has not been studied.
13. Nonclinical Toxicology
14. Clinical Studies
14.1 BRCA-mutated Metastatic Castration-Resistant Prostate Cancer (mCRPC)
The efficacy of AKEEGA was investigated in Cohort 1 of MAGNITUDE (NCT03748641), a randomized double-blind, placebo-controlled, multi-cohort, multi-center study in which 423 patients with homologous recombination repair (HRR) gene-mutated (HRRm) mCRPC were randomized (1:1) to receive niraparib 200 mg and abiraterone 1,000 mg (N=212) or placebo and abiraterone (N=211) until unacceptable toxicity or progression. All patients received prednisone 10 mg daily and a GnRH analog or had prior bilateral orchiectomy. Patients with mCRPC who had not received prior systemic therapy in the mCRPC setting except for a short duration of prior abiraterone acetate plus prednisone (up to four months) and ongoing ADT, were eligible. Patients could have received prior docetaxel or androgen-receptor (AR) targeted therapies in either the metastatic castration-sensitive prostate cancer (mCSPC) or non-metastatic castration-resistant prostate cancer (nmCRPC) setting.
Randomization was stratified by prior docetaxel for mCSPC (yes or no), prior AR targeted therapy for mCSPC or nmCRPC (yes or no), prior abiraterone acetate for mCRPC (yes or no), and BRCA-status ( BRCAm vs. other).
Of the 423 patients enrolled, 225 (53%) had BRCAgene mutations ( BRCAm). Mutation status of BRCAgenes was determined prospectively using the Foundation One CDx tissue assay or other clinical trial assays.
Among the 225 patients with BRCAm, the median age was 68 years (range 43–100) and 66% were 65 years of age or older; 72% were White, 17% Asian, and 1% Black, and 10% other or not reported; 12% were Hispanic or Latino; and baseline ECOG performance status (PS) was 0 (66%) or 1 (34%). Twenty-four percent had received prior docetaxel, 5% received prior AR-targeted therapy for mCSPC or nmCRPC, and 26% received prior abiraterone acetate plus prednisone for up to 4 months for mCRPC. Thirty-seven percent had bone-only metastases and 21% had visceral metastases. Seven percent had BRCA1 mutations, 78% had BRCA2 mutations, and 15% had BRCAmutations in combination with mutations in other HRR genes.
The major efficacy outcome measure was radiographic progression free survival (rPFS) determined by blinded independent central radiology (BICR) review evaluated per Response Evaluation Criteria In Solid Tumors (RECIST) 1.1 (soft tissue lesions) and Prostate Cancer Working Group-3 (PCWG-3) criteria (bone lesions). Overall survival (OS) was an additional efficacy outcome measure.
A statistically significant improvement in rPFS for niraparib plus abiraterone compared to placebo plus abiraterone was observed in BRCAm patients, and the Cohort 1 intention to treat (ITT) population. In an exploratory analysis in the subgroup of 198 (47%) patients with non- BRCAmutations, the rPFS hazard ratio was 0.99 (95% CI: 0.67, 1.44) and the OS hazard ratio was 1.13 (95% CI: 0.77, 1.64), indicating that the improvement in the ITT population was primarily attributed to the results seen in the subgroup of patients with BRCAm.
The efficacy results are presented in Table 4 and Figures 1 and 2 for patients in Cohort 1 with BRCAmutations.
| Endpoints | AKEEGA
(N=113) | Placebo
(N=112) |
|---|---|---|
| NE = not estimable | ||
|
||
| Radiographic Progression-free Survival * | ||
| Event of disease progression or death (%) | 45 (40%) | 64 (57%) |
| Median, months (95% CI) | 16.6 (13.9, NE) | 10.9 (8.3, 13.8) |
| Hazard Ratio †(95% CI) | 0.53 (0.36, 0.79) | |
| p-value ‡ | 0.0014 | |
At the protocol pre-specified final OS analysis in Cohort 1, 60 (53%) deaths and 70 (63%) deaths were observed in the AKEEGA arm and placebo arm, respectively, for patients with BRCAm. In an exploratory OS analysis in the subgroup of patients with BRCAm, the median in the AKEEGA arm was 30.4 (95% CI: 27.6, NE) and 28.6 months (95% CI: 23.8, 33.0) in the placebo arm, with an OS hazard ratio of 0.79 (95% CI: 0.55, 1.12).
Figure 1: Kaplan-Meier Plot of BICR Assessed Radiographic Progression-Free Survival in the BRCAm Population (MAGNITUDE, primary analysis)
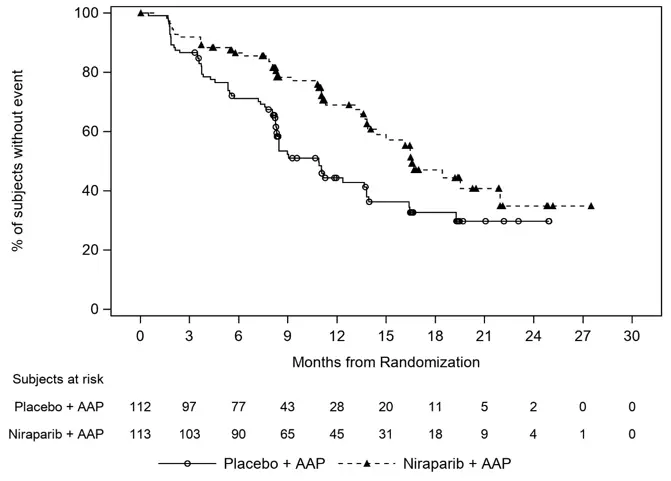
Figure 2: Kaplan-Meier Plot of Overall Survival in the BRCAm Population (MAGNITUDE, final analysis)

16. How is Akeega supplied
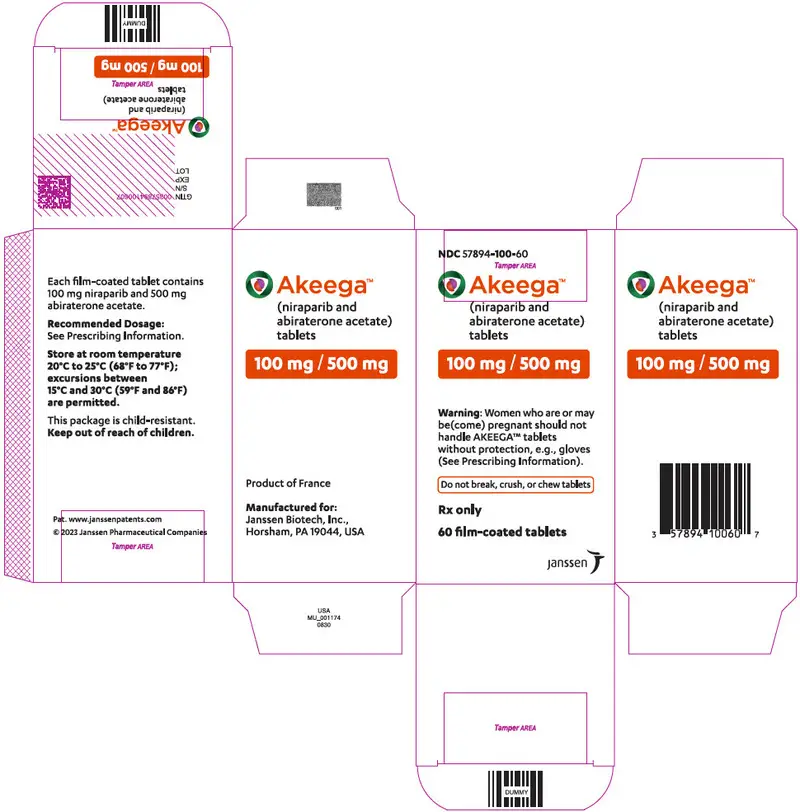
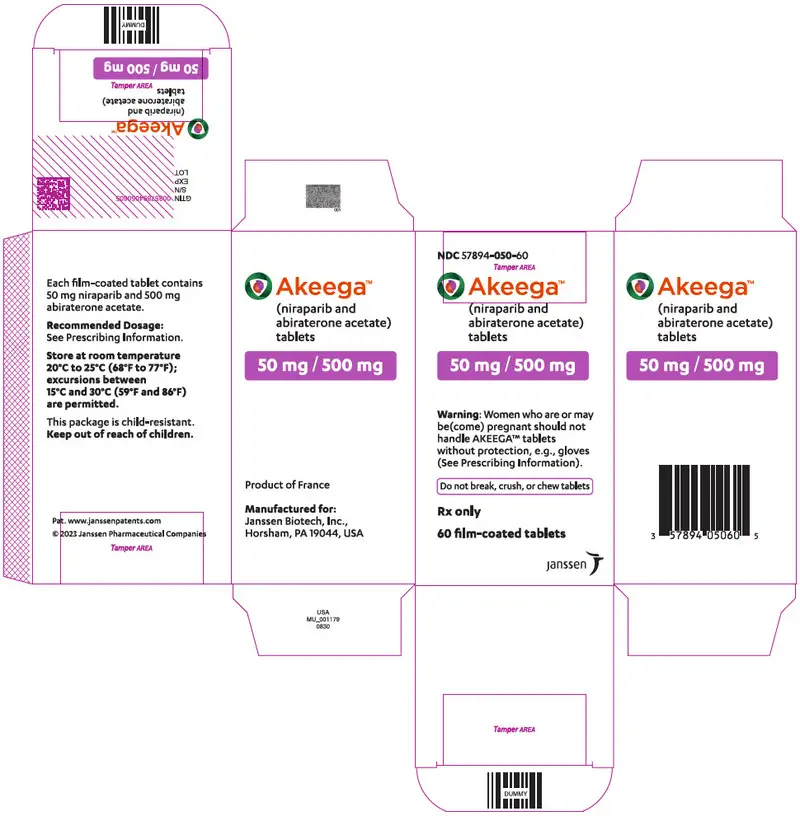
AKEEGA™ (niraparib and abiraterone acetate) tablets are available in the strengths and packages listed below:
- AKEEGA 50 mg/500 mg film-coated tablets
Yellowish orange to yellowish brown, oval, film-coated tablets debossed with "N 50 A" on one side and plain on the other side. They are available in bottles of 60 tablets.
NDC 57894-050-60 - AKEEGA 100 mg/500 mg film-coated tablets
Orange, oval, film-coated tablets debossed with "N 100 A" on one side and plain on the other side. They are available in bottles of 60 tablets.
NDC 57894-100-60
| AKEEGA
niraparib tosylate monohydrate and abiraterone acetate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AKEEGA
niraparib tosylate monohydrate and abiraterone acetate tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Janssen Biotech, Inc. (099091753) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanghai SynTheAll Pharmaceutical Co., Ltd. | 545342792 | api manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Changzhou SynTheAll Pharmaceutical Co., Ltd. | 544385021 | api manufacture(57894-100, 57894-050) , manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jiangsu Jiaerke Pharmaceuticals Group Corp., Ltd. | 527224249 | api manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zhejiang Xianju Junye Pharmaceutical Co., Ltd. | 421340422 | api manufacture(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | manufacture(57894-100, 57894-050) , pack(57894-100, 57894-050) , label(57894-100, 57894-050) , analysis(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 400345889 | api manufacture(57894-100, 57894-050) , manufacture(57894-100, 57894-050) , analysis(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon France S.A.S | 543127229 | analysis(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Cilag SpA | 542797928 | pack(57894-100, 57894-050) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Johnson & Johnson Private Limited | 677603030 | analysis(57894-100, 57894-050) | |