Drug Detail:Albuminar-25 (human) (Albumin (human) [ al-bue-min ])
Drug Class: Plasma expanders
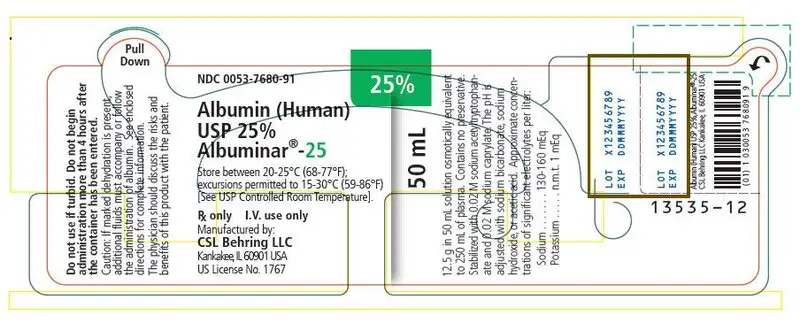
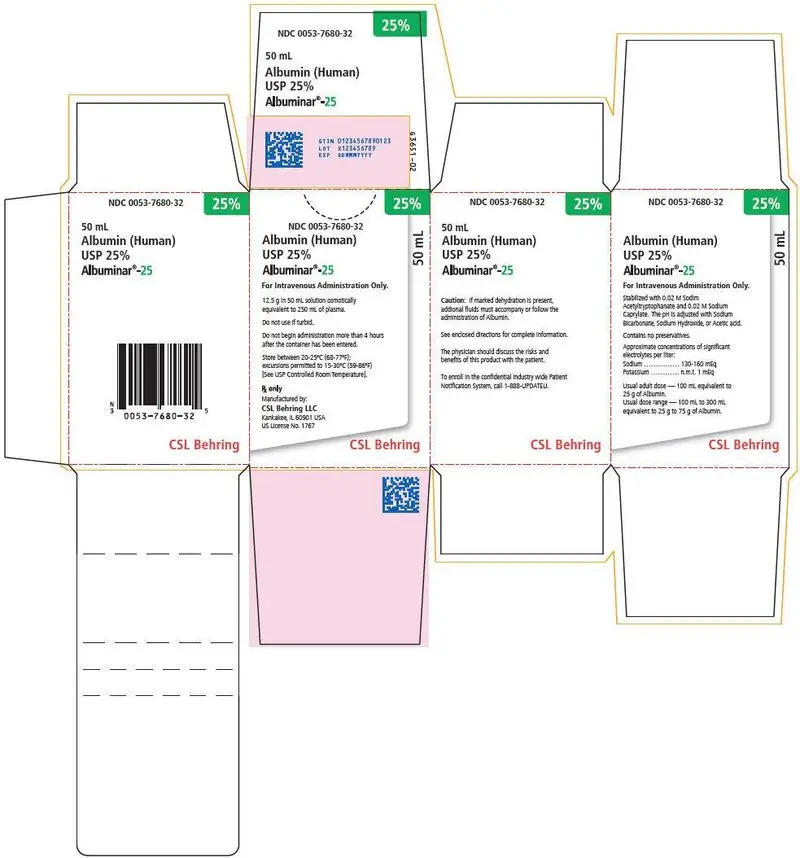
Albuminar-25 Description
Albumin (Human) 25%, ALBUMINAR®-25 is a sterile aqueous solution of albumin obtained from large pools of adult human venous plasma by low temperature controlled fractionation according to the Cohn process. It is stabilized with 0.02 M sodium acetyltryptophanate and 0.02 M sodium caprylate and pasteurized at 60°C for 10 hours.
All Source Plasma used in the manufacture of this product was tested by FDA-licensed Nucleic Acid Testing (NAT) for HBV, HCV, and HIV-1 and found to be nonreactive (negative).
ALBUMINAR®-25 is a solution containing in each 100 mL, 25 grams of serum albumin, osmotically equivalent to 500 mL of normal human plasma. The pH of the solution is adjusted with sodium bicarbonate, sodium hydroxide, or acetic acid. Approximate concentrations of significant electrolytes per liter are: sodium - 130-160 mEq; and potassium - n.m.t. 1 mEq. The solution contains no preservative. This product has been prepared in accordance with the requirements established by the Food and Drug Administration and is in compliance with the standards of the United States Pharmacopeia.
ALBUMINAR®-25 is to be administered by the intravenous route.
The heat treatment step employed in the manufacture of ALBUMINAR®-25 pasteurization of the final container at 60°C for 10 hours, has been validated in a series of in vitro experiments for its capacity to inactivate Human Immunodeficiency Virus type 1 (HIV-1), and the following model viruses: Bovine Viral Diarrhea Virus (BVDV - an enveloped virus used as a model for hepatitis C virus), Pseudorabies (PrV - a large, enveloped virus), and Encephalomyocarditis Virus (EMC - a small non-enveloped virus). For each virus studied, three independent experiments were conducted using Albumin (Human) 5%, ALBUMINAR®-5 and ALBUMINAR®-25 with the results provided in Table 1.1
| Pasteurization (60°C for 10 hours) Viral Reduction Studies (log10 reduction) |
|
| Virus | Albumin (Human) 5%, ALBUMINAR®-5 |
| HIV-1 | >5.44, >6.38 and >6.31 |
| BVDV | >6.01, >6.76 and >6.55 |
| PrV | >7.30, >7.68 and >7.63 |
| EMC | >7.38, >7.97 and >7.97 |
| Virus | Albumin (Human) 25%, ALBUMINAR®-25 |
| HIV-1 | >5.50, >6.57 and >6.64 |
| BVDV | >5.99, >5.81 and >5.32 |
| PrV | >7.32, >7.20 and >7.42 |
| EMC | >7.10, >7.89 and >7.87 |
| ALBUMINAR-25
albumin (human) solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - CSL Behring LLC (058268293) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring LLC | 058268293 | MANUFACTURE | |