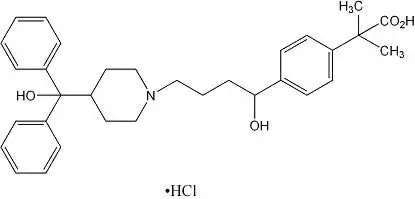
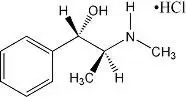
Overdosage
Most reports of fexofenadine hydrochloride overdose contain limited information. However, dizziness, drowsiness, and dry mouth have been reported. For the pseudoephedrine hydrochloride component of FEXOFENADINE HCl AND PSEUDOEPHEDRINE HCl , information on acute overdose is limited to the marketing history of pseudoephedrine hydrochloride. Single doses of fexofenadine hydrochloride up to 800 mg (6 healthy volunteers at this dose level), and doses up to 690 mg twice daily for one month (3 healthy volunteers at this dose level), were administered without the development of clinically significant adverse events.
In large doses, sympathomimetics may give rise to giddiness, headache, nausea, vomiting, sweating, thirst, tachycardia, precordial pain, palpitations, difficulty in micturition, muscular weakness and tenseness, anxiety, restlessness, and insomnia. Many patients can present a toxic psychosis with delusions and hallucinations. Some may develop cardiac arrhythmias, circulatory collapse, convulsions, coma, and respiratory failure.
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Following administration of terfenadine, hemodialysis did not effectively remove fexofenadine, the major active metabolite of terfenadine, from blood (up to 1.7% removed). The effect of hemodialysis on the removal of pseudoephedrine is unknown.
No deaths occurred in mature mice and rats at oral doses of fexofenadine hydrochloride up to 5000 mg/kg (approximately 170 and 340 times, respectively, the maximum recommended human daily oral dose of FEXOFENADINE HCl AND PSEUDOEPHEDRINE HCl on a mg/m2 basis.) The median oral lethal dose in newborn rats was 438 mg/kg (approximately 30 times the maximum recommended human daily oral dose of FEXOFENADINE HCl AND PSEUDOEPHEDRINE HCl on a mg/m2 basis). In dogs, no evidence of toxicity was observed at oral doses up to 2000 mg/kg (approximately 450 times the maximum recommended human daily oral dose on a mg/m2 basis). The oral median lethal dose of pseudoephedrine hydrochloride in rats was 1674 mg/kg (approximately 55 times the maximum recommended human daily oral dose of ALLEGRA-D 12 HOUR on a mg/m2 basis).