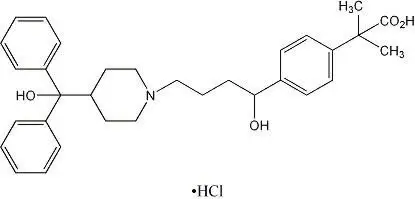
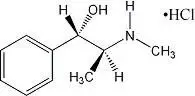
Drug Detail:Allegra-d 24 hour (Fexofenadine and pseudoephedrine [ fex-oh-fen-a-deen-and-soo-doe-ee-fed-rin ])
Drug Class: Upper respiratory combinations
Related/similar drugs
prednisone, fluticasone nasal, cetirizine, loratadine, promethazine, ZyrtecAllegra-D 24 Hour - Clinical Pharmacology
Pharmacokinetics
The pharmacokinetics of fexofenadine hydrochloride in subjects with seasonal allergic rhinitis were similar to those in healthy volunteers.
Contraindications
ALLEGRA-D 24 HOUR is contraindicated in patients with known hypersensitivity to any of its ingredients.
Due to its pseudoephedrine component, ALLEGRA-D 24 HOUR is contraindicated in patients with narrow-angle glaucoma or urinary retention, and in patients receiving monoamine oxidase (MAO) inhibitor therapy or within fourteen (14) days of stopping such treatment (see Drug Interactions section). It is also contraindicated in patients with severe hypertension, or severe coronary artery disease, and in those who have shown idiosyncrasy to its components, to adrenergic agents, or to other drugs of similar chemical structures. Manifestations of patient idiosyncrasy to adrenergic agents include: insomnia, dizziness, weakness, tremor, or arrhythmias.
Warnings
Sympathomimetic amines should be used with caution in patients with hypertension, diabetes mellitus, ischemic heart disease, increased intraocular pressure, hyperthyroidism, renal impairment, or prostatic hypertrophy (see CONTRAINDICATIONS). Sympathomimetic amines may produce central nervous system stimulation with convulsions or cardiovascular collapse with accompanying hypotension.
Precautions
Drug Interactions
Fexofenadine hydrochloride and pseudoephedrine hydrochloride do not influence the pharmacokinetics of each other when administered concomitantly.
Fexofenadine has been shown to exhibit minimal (ca. 5%) metabolism. However, co-administration of fexofenadine hydrochloride with either ketoconazole or erythromycin led to increased plasma concentrations of fexofenadine. Fexofenadine had no effect on the pharmacokinetics of either erythromycin or ketoconazole. In 2 separate studies, fexofenadine hydrochloride 120 mg twice daily was co-administered with either erythromycin 500 mg every 8 hours or ketoconazole 400 mg once daily under steady-state conditions to healthy volunteers (n=24, each study). No differences in adverse events or QTc interval were observed when subjects were administered fexofenadine hydrochloride alone or in combination with either erythromycin or ketoconazole. The findings of these studies are summarized in the following table:
| Concomitant Drug | CmaxSS (Peak plasma concentration) | AUCss(0–12h)
(Extent of systemic exposure) |
|---|---|---|
| Erythromycin (500 mg every 8 hrs) | +82% | +109% |
| Ketoconazole (400 mg once daily) | +135% | +164% |
The changes in plasma levels were within the range of plasma levels achieved in adequate and well-controlled clinical trials.
The mechanism of these interactions has been evaluated in in vitro, in situ, and in vivo animal models. These studies indicate that ketoconazole or erythromycin co-administration enhances fexofenadine gastrointestinal absorption. This observed increase in the bioavailability of fexofenadine may be due to transport-related effects, such as p-glycoprotein. In vivo animal studies also suggest that in addition to enhancing absorption, ketoconazole decreases fexofenadine gastrointestinal secretion, while erythromycin may also decrease biliary excretion.
Due to the pseudoephedrine component, ALLEGRA-D 24 HOUR is contraindicated in patients taking monoamine oxidase inhibitors and for 14 days after stopping use of an MAO inhibitor. Concomitant use with antihypertensive drugs which interfere with sympathetic activity (e.g., methyldopa, mecamylamine, and reserpine) may reduce their antihypertensive effects. Increased ectopic pacemaker activity can occur when pseudoephedrine is used concomitantly with digitalis. Care should be taken in the administration of ALLEGRA-D 24 HOUR concomitantly with other sympathomimetic amines because combined effects on the cardiovascular system may be harmful to the patient (see WARNINGS).
Allegra-D 24 Hour Dosage and Administration
The recommended dose of ALLEGRA-D 24 HOUR Extended-Release Tablets is one tablet once daily administered on an empty stomach with water for adults and children 12 years of age and older. ALLEGRA-D 24 HOUR tablets should generally be avoided in patients with renal insufficiency. ALLEGRA-D 24 HOUR must be swallowed whole and never crushed or chewed.
| ALLEGRA--D 24 HOUR
fexofenadine hydrochloride and pseudoephedrine hydrochloride tablet, film coated |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (783243835) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanofi-aventis U.S. LLC | 783243835 | MANUFACTURE | |