Drug Detail:Allegra (Fexofenadine)
Drug Class: Antihistamines
Highlights of Prescribing Information
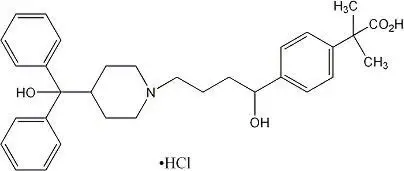
ALLEGRA (fexofenadine hydrochloride) tablet, orally disintegrating for oral use
ALLEGRA (fexofenadine hydrochloride) tablet, film coated for oral use
ALLEGRA (fexofenadine hydrochloride) suspension for oral use
Initial U.S. Approval: 1996
Recent Major Changes
| Dosage and Administration, ALLEGRA ODT (2.2) | [7/2007] |
| Dosage and Administration, ALLEGRA oral suspension(2.3) | [10/2006] |
Indications and Usage for Allegra
ALLEGRA is an H1-receptor antagonist indicated for:
- Relief of symptoms associated with seasonal allergic rhinitis in patients ≥ 2 years of age (1.1)
- Treatment of uncomplicated skin manifestations of chronic idiopathic urticaria in patients ≥ 6 months of age. (1.2)
Allegra Dosage and Administration
| Patient Population | ALLEGRA tablets (2.1) | ALLEGRA ODT (2.2) | ALLEGRA oral suspension (2.3) |
|---|---|---|---|
|
|||
| Adults and children ≥ 12 years | 60 mg twice daily* or 180 mg once daily † | N/A | N/A |
| Children 6 to 11 years | 30 mg twice daily* | 30 mg twice daily* | 30 mg twice daily* |
| Children 2 to 5 years | N/A | N/A | 30 mg twice daily* |
| Children 6 months to less than 2 years | N/A | N/A | 15 mg twice daily*, ‡ |
-
ALLEGRA tablets take with water (2.1)
-
ALLEGRA ODT: take on an empty stomach: allow to disintegrate on the tongue and swallow with or without water; do not remove from original blister package until time of administration; do not break or use partial tablets (2.2)
Dosage Forms and Strengths
- ALLEGRA tablets: 30 mg, 60 mg, and 180 mg (3)
- ALLEGRA ODT: 30 mg (3)
- ALLEGRA oral suspension: 30 mg/5 mL (6 mg/mL) (3)
Contraindications
- Patients with known hypersensitivity to fexofenadine and any of the ingredients of ALLEGRA(4)
Warnings and Precautions
- ALLEGRA ODT contains phenylalanine, a component of aspartame. Other ALLEGRA products do not contain phenylalanine.
Adverse Reactions/Side Effects
The most common adverse reactions (≥ 2%) in subjects age 12 years and older were headache, back pain, dizziness, stomach discomfort, and pain in extremity. In subjects aged 6 to 11 years, cough, upper respiratory tract infection, pyrexia and otitis media were more frequently reported. In subjects aged 6 months to 5 years, vomiting, diarrhea, somnolence/fatigue and rhinorrhea were more frequently reported. (6.1) Other adverse reactions have been reported. (6)
To report SUSPECTED ADVERSE REACTIONS, contact sanofi-aventis at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
- Antacids: Do not take at the same time as aluminum and magnesium containing antacids (7.1)
- Fruit juice: Take with water; not fruit juice
Use In Specific Populations
- Pregnancy: Use only if benefit justifies risk to fetus (8.1)
- Nursing Mothers: Use with caution (8.3)
See 17 for PATIENT COUNSELING INFORMATION
Revised: 3/2012
Full Prescribing Information
1. Indications and Usage for Allegra
2. Allegra Dosage and Administration
3. Dosage Forms and Strengths
ALLEGRA tablets are available in 30 mg, 60 mg, and 180 mg strengths. ALLEGRA tablets are coated with a peach colored film coating. Tablets have the following unique shape and identifiers: 30 mg tablets are round, bi-convex and have 03 on one side and a scripted "e" on the other; 60 mg tablets are oval, bi-convex and have 06 on one side and a scripted "e" on the other; and 180 mg tablets are oblong, bi-convex and have 018 on one side and a scripted "e" on the other.
ALLEGRA ODT is available as a 30 mg orally disintegrating tablet and is white, flat-faced, ½-inch round shaped with beveled edges and debossed with a scripted "e" on one side and "311AV" on the other side.
ALLEGRA oral suspension is available as 30 mg/ 5 mL (6 mg/mL).
4. Contraindications
ALLEGRA tablets, ALLEGRA ODT and ALLEGRA oral suspension are contraindicated in patients with known hypersensitivity to fexofenadine and any of the ingredients of ALLEGRA. Rare cases of hypersensitivity reactions with manifestations such as angioedema, chest tightness, dyspnea, flushing and systemic anaphylaxis have been reported.
6. Adverse Reactions/Side Effects
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure to fexofenadine hydrochloride in 5083 patients in trials for allergic rhinitis and chronic idiopathic urticaria. In these trials, 3010 patients 12 years of age and older with seasonal allergic rhinitis were exposed to fexofenadine hydrochloride at doses of 20 to 240 mg twice daily or 120 to 180 mg once daily. A total of 646 patients 6 to 11 years of age with seasonal allergic rhinitis were exposed to fexofenadine hydrochloride at doses of 15 to 60 mg twice daily. The duration of treatment in these trials was 2 weeks. A total of 534 patients 6 months to 5 years of age with allergic rhinitis were exposed to fexofenadine hydrochloride at doses of 15 to 30 mg twice daily. The duration of treatment in these trials ranged from 1 day to 2 weeks. There were 893 patients 12 years of age and older with chronic idiopathic urticaria exposed to fexofenadine hydrochloride at doses of 20 to 240 mg twice daily or 180 mg once daily. The duration of treatment in these trials was 4 weeks.
7. Drug Interactions
7.2 Erythromycin and Ketoconazole
Fexofenadine has been shown to exhibit minimal (ca. 5%) metabolism. However, co–administration of fexofenadine hydrochloride with either ketoconazole or erythromycin led to increased plasma concentrations of fexofenadine in healthy adult subjects. Fexofenadine had no effect on the pharmacokinetics of either erythromycin or ketoconazole. In 2 separate studies in healthy adult subjects, fexofenadine hydrochloride 120 mg twice daily (240 mg total daily dose) was co-administered with either erythromycin 500 mg every 8 hours or ketoconazole 400 mg once daily under steady-state conditions to healthy adult subjects (n=24, each study). No differences in adverse events or QTc interval were observed when subjects were administered fexofenadine hydrochloride alone or in combination with either erythromycin or ketoconazole. The findings of these studies are summarized in the following table
| Concomitant Drug | CmaxSS
(Peak plasma concentration) | AUCss(0–12h)
(Extent of systemic exposure) |
|---|---|---|
| Erythromycin (500 mg every 8 hrs) | +82% | +109% |
| Ketoconazole (400 mg once daily) | +135% | +164% |
The changes in plasma levels were within the range of plasma levels achieved in adequate and well-controlled clinical trials.
The mechanism of these interactions has been evaluated in in vitro, in situ, and in vivo animal models. These studies indicate that ketoconazole or erythromycin co-administration enhances fexofenadine gastrointestinal absorption. This observed increase in the bioavailability of fexofenadine may be due to transport-related effects, such as p-glycoprotein. In vivo animal studies also suggest that in addition to enhancing absorption, ketoconazole decreases fexofenadine gastrointestinal secretion, while erythromycin may also decrease biliary excretion.
8. Use In Specific Populations
8.3 Nursing Mothers
It is not known if fexofenadine is excreted in human milk. There are no adequate and well-controlled studies in women during lactation. Because many drugs are excreted in human milk, caution should be exercised when fexofenadine hydrochloride is administered to a nursing woman.
8.4 Pediatric Use
The recommended doses of fexofenadine hydrochloride in pediatric patients 6 months to 11 years of age are based on cross-study comparison of the pharmacokinetics of fexofenadine in adults and pediatric subjects and on the safety profile of fexofenadine hydrochloride in both adult and pediatric subjects at doses equal to or higher than the recommended doses.The safety and effectiveness of fexofenadine hydrochloride in pediatric patients under 6 months of age have not been established.
The safety of fexofenadine hydrochloride is based on the administration of ALLEGRA tablets at a dose of 30 mg twice daily demonstrated in 438 pediatric subjects 6 years to 11 years of age in 2 placebo-controlled 2-week seasonal allergic rhinitis trials. The safety of fexofenadine hydrochloride at doses of 15mg and 30 mg given once and twice a day has been demonstrated in 969 pediatric subjects (6 months to 5 years of age) with allergic rhinitis in 3 pharmacokinetic studies and 3 safety studies. The safety of fexofenadine hydrochloride for the treatment of chronic idiopathic urticaria in subjects 6 months to 11 years of age is based on cross-study comparison of the pharmacokinetics of ALLEGRA in adult and pediatric subjects and on the safety profile of fexofenadine in both adult and pediatric subjects at doses equal to or higher than the recommended dose.
The effectiveness of fexofenadine hydrochloride for the treatment of seasonal allergic rhinitis in subjects 6 to 11 years of age was demonstrated in 1 trial (n=411) in which ALLEGRA tablets 30 mg twice daily significantly reduced total symptom scores compared to placebo, along with extrapolation of demonstrated efficacy in subjects aged 12 years and above, and the pharmacokinetic comparisons in adults and children. The effectiveness of fexofenadine hydrochloride 30 mg twice daily for the treatment of seasonal allergic rhinitis in patients 2 to 5 years of age is based on the pharmacokinetic comparisons in adult and pediatric subjects and an extrapolation of the demonstrated efficacy of fexofenadine hydrochloride in adult subjects with this condition and the likelihood that the disease course, pathophysiology, and the drug's effect are substantially similar in pediatric patients to those in adult patients. The effectiveness of fexofenadine hydrochloride for the treatment of chronic idiopathic urticaria in patients 6 months to 11 years of age is based on the pharmacokinetic comparisons in adults and children and an extrapolation of the demonstrated efficacy of ALLEGRA in adults with this condition and the likelihood that the disease course, pathophysiology and the drug's effect are substantially similar in children to that of adult patients. Administration of a 15 mg dose of fexofenadine hydrochloride to pediatric subjects 6 months to less than 2 years of age and a 30 mg dose to pediatric subjects 2 to 11 years of age produced exposures comparable to those seen with a dose of 60 mg administered to adults.
12. Allegra - Clinical Pharmacology
12.3 Pharmacokinetics
The pharmacokinetics of fexofenadine hydrochloride in subjects with seasonal allergic rhinitis and subjects with chronic urticaria were similar to those in healthy subjects.
17 PATIENT COUNSELING INFORMATION
Provide the following information to patients and parents/caregivers of pediatric patients taking ALLEGRA tablets, ALLEGRA ODT or ALLEGRA oral suspension:
-
ALLEGRA tablets, ALLEGRA ODT or ALLEGRA oral suspension are prescribed for the relief of symptoms of seasonal allergic rhinitis or for the relief of symptoms of chronic idiopathic urticaria (hives). Instruct patients to take ALLEGRA only as prescribed. Do not exceed the recommended dose.If any untoward effects occur while taking ALLEGRA, discontinue use and consult a doctor.
-
Patients who are hypersensitive to any of the ingredients should not use these products.
-
Patients who are pregnant or nursing should use these products only if the potential benefit justifies the potential risk to the fetus or nursing infant.
-
Advise patients and parents/caregivers of pediatric patients to store the medication in a tightly closed container in a cool, dry place, away from small children.
-
Advise patients and parents/caregivers not to take ALLEGRA with fruit juices.
For ALLEGRA tablets: Advise patients to take the ALLEGRA tablets with water.
For ALLEGRA ODT: Advise patients to take their dose on an empty stomach. Allow ALLEGRA ODT to disintegrate on the tongue before swallowing, with or without water. ALLEGRA ODT is not intended to be chewed. Store ALLEGRA ODT in its original blister package. Do not remove ALLEGRA ODT from the original blister package until the time of administration.
Phenylketonurics: ALLEGRA ODT contains phenylalanine, a component of aspartame. Each 30-mg ALLEGRA ODT contains 5.3 mg phenylalanine. ALLEGRA products other than ALLEGRA ODT do not contain phenylalanine.
For ALLEGRA oral suspension: Advise patients and parents/caregivers of pediatric patients to shake the ALLEGRA oral suspension bottle well, before each use.
| ALLEGRA
fexofenadine hydrochloride tablet, orally disintegrating |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ALLEGRA
fexofenadine hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| ALLEGRA
fexofenadine hydrochloride tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ALLEGRA
fexofenadine hydrochloride tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| ALLEGRA
fexofenadine hydrochloride suspension |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - sanofi-aventis U.S. LLC (783243835) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cima Labs Inc. | 173625823 | MANUFACTURE(0088-1113) , ANALYSIS(0088-1113) , LABEL(0088-1113) , PACK(0088-1113) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanofi-aventis U.S. LLC | 783243835 | MANUFACTURE(0088-1106, 0088-1107, 0088-1109, 0088-1097) , ANALYSIS(0088-1106, 0088-1107, 0088-1109, 0088-1097) , LABEL(0088-1106, 0088-1107, 0088-1109, 0088-1097) , PACK(0088-1106, 0088-1107, 0088-1109, 0088-1097) | |