Drug Detail:Alphagan p eye drops (Brimonidine ophthalmic [ bri-mo-ni-deen-off-thal-mik ])
Drug Class: Ophthalmic glaucoma agents
Highlights of Prescribing Information
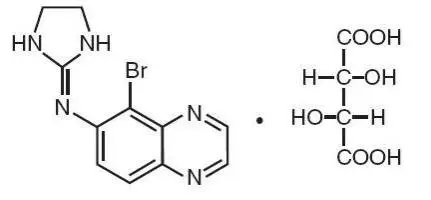
ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.1% and 0.15%
Initial U.S. Approval: 1996
Indications and Usage for Alphagan P
ALPHAGAN® P is an alpha adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. (1)
Alphagan P Dosage and Administration
One drop in the affected eye(s), three times daily, approximately 8 hours apart. (2)
Dosage Forms and Strengths
Solution containing 1 or 1.5 mg/mL brimonidine tartrate. (3)
Contraindications
Neonates and infants (under the age of 2 years). (4.1)
Warnings and Precautions
Potentiation of vascular insufficiency. (5.1)
Adverse Reactions/Side Effects
Most common adverse reactions occurring in approximately 5% to 20% of patients receiving brimonidine ophthalmic solution (0.1%-0.2%) included allergic conjunctivitis, burning sensation, conjunctival folliculosis, conjunctival hyperemia, eye pruritus, hypertension, ocular allergic reaction, oral dryness, and visual disturbance. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-433-8871 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antihypertensives/cardiac glycosides may lower blood pressure. (7.1)
- Use with CNS depressants may result in an additive or potentiating effect. (7.2)
- Tricyclic antidepressants may potentially blunt the hypotensive effect of systemic clonidine. (7.3)
- Monoamine oxidase inhibitors may result in increased hypotension. (7.4)
Use In Specific Populations
Use with caution in children ≥ 2 years of age. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2013
Related/similar drugs
latanoprost ophthalmic, epinephrine ophthalmic, timolol ophthalmic, brimonidine ophthalmic, pilocarpine ophthalmic, Lumigan, XalatanFull Prescribing Information
1. Indications and Usage for Alphagan P
ALPHAGAN® P (brimonidine tartrate ophthalmic solution) 0.1% or 0.15% is an alpha adrenergic receptor agonist indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
2. Alphagan P Dosage and Administration
The recommended dose is one drop of ALPHAGAN® P in the affected eye(s) three times daily, approximately 8 hours apart. ALPHAGAN® P ophthalmic solution may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic product is to be used, the different products should be instilled at least 5 minutes apart.
4. Contraindications
5. Warnings and Precautions
5.1 Potentiation of Vascular Insufficiency
ALPHAGAN® P may potentiate syndromes associated with vascular insufficiency. ALPHAGAN® P should be used with caution in patients with depression, cerebral or coronary insufficiency, Raynaud’s phenomenon, orthostatic hypotension, or thromboangiitis obliterans.
5.3 Contamination of Topical Ophthalmic Products After Use
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface (see PATIENT COUNSELING INFORMATION, 17).
6. Adverse Reactions/Side Effects
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse reactions occurring in approximately 10-20% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: allergic conjunctivitis, conjunctival hyperemia, and eye pruritus. Adverse reactions occurring in approximately 5-9% included: burning sensation, conjunctival folliculosis, hypertension, ocular allergic reaction, oral dryness, and visual disturbance.
Adverse reactions occurring in approximately 1-4% of the subjects receiving brimonidine ophthalmic solution (0.1-0.2%) included: abnormal taste, allergic reaction, asthenia, blepharitis, blepharoconjunctivitis, blurred vision, bronchitis, cataract, conjunctival edema, conjunctival hemorrhage, conjunctivitis, cough, dizziness, dyspepsia, dyspnea, epiphora, eye discharge, eye dryness, eye irritation, eye pain, eyelid edema, eyelid erythema, fatigue, flu syndrome, follicular conjunctivitis, foreign body sensation, gastrointestinal disorder, headache, hypercholesterolemia, hypotension, infection (primarily colds and respiratory infections), insomnia, keratitis, lid disorder, pharyngitis, photophobia, rash, rhinitis, sinus infection, sinusitis, somnolence, stinging, superficial punctate keratopathy, tearing, visual field defect, vitreous detachment, vitreous disorder, vitreous floaters, and worsened visual acuity.
The following reactions were reported in less than 1% of subjects: corneal erosion, hordeolum, nasal dryness, and taste perversion.
7. Drug Interactions
7.1 Antihypertensives/Cardiac Glycosides
Because ALPHAGAN® P may reduce blood pressure, caution in using drugs such as antihypertensives and/or cardiac glycosides with ALPHAGAN® P is advised.
7.2 CNS Depressants
Although specific drug interaction studies have not been conducted with ALPHAGAN® P, the possibility of an additive or potentiating effect with CNS depressants (alcohol, barbiturates, opiates, sedatives, or anesthetics) should be considered.
7.3 Tricyclic Antidepressants
Tricyclic antidepressants have been reported to blunt the hypotensive effect of systemic clonidine. It is not known whether the concurrent use of these agents with ALPHAGAN® P in humans can lead to resulting interference with the IOP lowering effect. Caution is advised in patients taking tricyclic antidepressants which can affect the metabolism and uptake of circulating amines.
7.4 Monoamine Oxidase Inhibitors
Monoamine oxidase (MAO) inhibitors may theoretically interfere with the metabolism of brimonidine and potentially result in an increased systemic side-effect such as hypotension. Caution is advised in patients taking MAO inhibitors which can affect the metabolism and uptake of circulating amines.
8. Use In Specific Populations
8.4 Pediatric Use
ALPHAGAN® P is contraindicated in children under the age of 2 years (see CONTRAINDICATIONS, 4.1). During postmarketing surveillance, apnea, bradycardia, coma, hypotension, hypothermia, hypotonia, lethargy, pallor, respiratory depression, and somnolence have been reported in infants receiving brimonidine. The safety and effectiveness of brimonidine tartrate have not been studied in children below the age of 2 years.
In a well-controlled clinical study conducted in pediatric glaucoma patients (ages 2 to 7 years) the most commonly observed adverse reactions with brimonidine tartrate ophthalmic solution 0.2% dosed three times daily were somnolence (50-83% in patients ages 2 to 6 years) and decreased alertness. In pediatric patients 7 years of age (>20 kg), somnolence appears to occur less frequently (25%). Approximately 16% of patients on brimonidine tartrate ophthalmic solution discontinued from the study due to somnolence.
17. Patient Counseling Information
Patients should be instructed that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions (see WARNINGS AND PRECAUTIONS, 5.3). Always replace the cap after using. If solution changes color or becomes cloudy, do not use. Do not use the product after the expiration date marked on the bottle.
Patients also should be advised that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
As with other similar medications, ALPHAGAN® P may cause fatigue and/or drowsiness in some patients. Patients who engage in hazardous activities should be cautioned of the potential for a decrease in mental alertness.
© 2013 Allergan, Inc.
Irvine, CA 92612, U.S.A.
® marks owned by Allergan, Inc.
Patented. See: www.allergan.com/products/patent_notices
Made in the U.S.A.

71816US16
| ALPHAGAN P
brimonidine tartrate solution/ drops |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| ALPHAGAN P
brimonidine tartrate solution/ drops |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |