Drug Detail:Altuviiio (Antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl)
Drug Class: Miscellaneous coagulation modifiers
Highlights of Prescribing Information
ALTUVIIIO™ [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl], lyophilized powder for solution, for intravenous use
Initial U.S. Approval: 2023
Indications and Usage for Altuviiio
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment & control of bleeding episodes
- Perioperative management of bleeding (1)
Limitation of Use:
ALTUVIIIO is not indicated for the treatment of von Willebrand disease. (1)
Altuviiio Dosage and Administration
For intravenous use only.
- Each ALTUVIIIO vial label states Factor VIII activity in international units (IU or unit). (2.1)
- For routine prophylaxis: 50 IU/kg once weekly. (2.1)
- For on-demand treatment and control of bleeding episodes and perioperative management: 50 IU/kg (2.1)
Estimated Increment of Factor VIII (IU/dL or % of normal) = 50 IU/kg × 2 (IU/dL per IU/kg.) (2.1)
To achieve a specific target Factor VIII activity level, use the following formula: Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL). (2.1).
Dosage Forms and Strengths
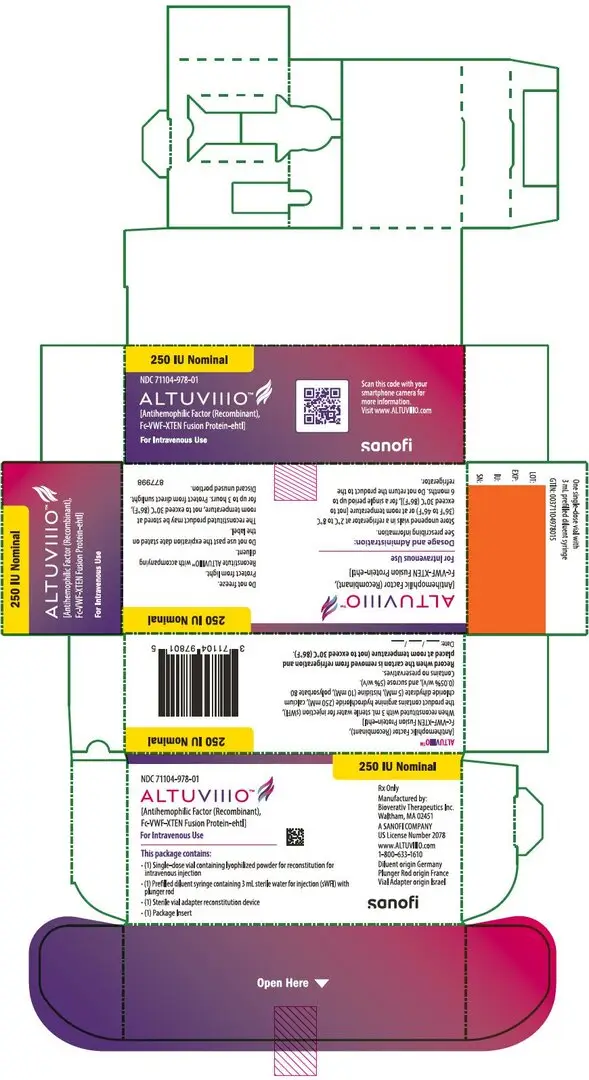
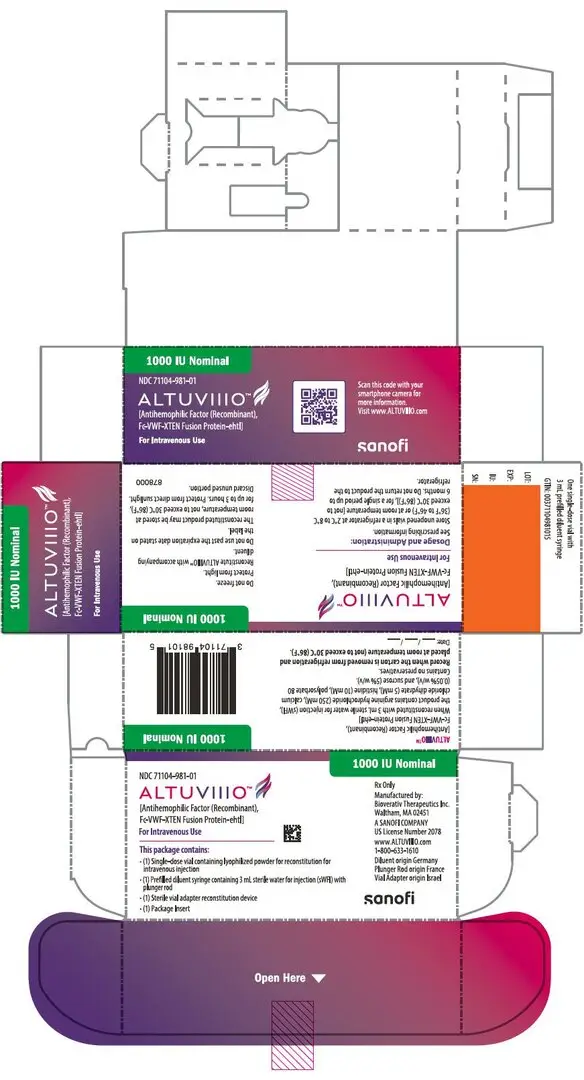
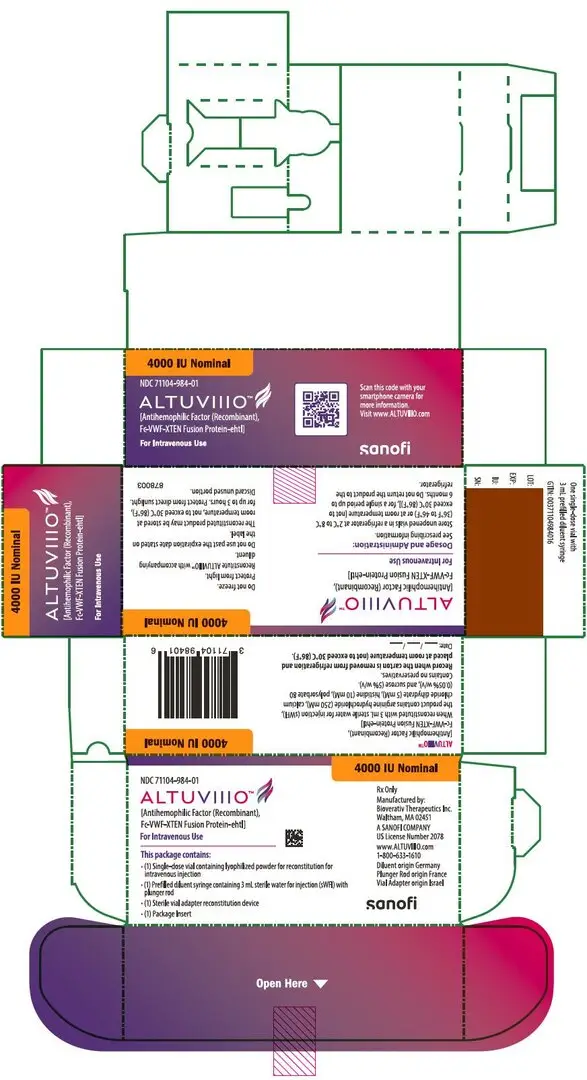
For injection: nominally 250, 500, 750, 1000, 2000, 3000, or 4000 IU, lyophilized powder in single-dose vials for reconstitution. (3)
Contraindications
Do not use in patients who have had severe hypersensitivity reactions, including anaphylaxis, to ALTUVIIIO or excipients of ALTUVIIIO. (4)
Warnings and Precautions
- Hypersensitivity reactions, including anaphylaxis, may occur. Should symptoms occur, immediately discontinue ALTUVIIIO and initiate appropriate treatment. (5.1)
- Neutralizing antibodies (inhibitors) to Factor VIII have been reported. If expected plasma Factor VIII activity levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures Factor VIII inhibitor concentration. (5.2, 5.3)
Adverse Reactions/Side Effects
Most common adverse reaction (incidence >10%) are headache and arthralgia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bioverativ Therapeutics Inc. (A SANOFI COMPANY) at 1-800-633-1610 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pediatric Use: No dosing adjustment is needed in this population. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
Related/similar drugs
tranexamic acid, desmopressin, DDAVP, Cyklokapron, antihemophilic factor, RoctavianFull Prescribing Information
1. Indications and Usage for Altuviiio
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a von Willebrand Factor (VWF) independent recombinant DNA-derived, Factor VIII concentrate indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- Routine prophylaxis to reduce the frequency of bleeding episodes
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
2. Altuviiio Dosage and Administration
For intravenous use after reconstitution only.
2.1 Dose
- Each ALTUVIIIO vial label states the Factor VIII potency in international units (IU). One IU corresponds to the Factor VIII activity contained in one milliliter of normal human plasma, as defined by the current World Health Organization (WHO) international standard for Factor VIII concentrate.
- Potency assignment for ALTUVIIIO is determined using an activated partial thromboplastin time (aPTT)-based one-stage clotting assay. It is recommended to use a validated one-stage clotting assay to measure ALTUVIIIO Factor VIII activity in plasma. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid based aPTT reagent in one-stage clotting assay by approximately 2.5-fold [see Warnings and Precautions (5.3)].
For the dose of 50 IU/kg, the expected in vivo peak increase in Factor VIII level expressed as IU/dL (or % of normal) is estimated using the following formula:
Estimated Increment of Factor VIII (IU/dL or % of normal) = 50 IU/kg × 2 (IU/dL per IU/kg)
To achieve a specific target Factor VIII activity level, use the following formula: Dosage (IU) = Body Weight (kg) × Desired Factor VIII Increase (IU/dL or % normal) × 0.5 (IU/kg per IU/dL).
3. Dosage Forms and Strengths
ALTUVIIIO is available as a white to off-white lyophilized powder for reconstitution in single-dose vials containing nominally 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU) per vial.
4. Contraindications
ALTUVIIIO is contraindicated in patients who have had severe hypersensitivity reactions, including anaphylaxis, to the product or its excipients [see Description (11)].
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, may occur with ALTUVIIIO. Allergic-type hypersensitivity reactions were not reported in the clinical trials. Inform patients of signs of hypersensitivity reactions that may progress to anaphylaxis (including hives, shortness of breath, chest tightness, wheezing, hypotension and itching). Advise patients to discontinue use of ALTUVIIIO if hypersensitivity symptoms occur and contact a physician and/or seek immediate emergency care.
5.2 Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to Factor VIII are possible following administration of ALTUVIIIO. Neutralizing antibodies were not reported in the clinical trials. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If the patient's plasma Factor VIII level fails to increase as expected or if bleeding is not controlled after ALTUVIIIO administration, the presence of an inhibitor (neutralizing antibodies) should be suspected, and appropriate testing performed [see Warnings and Precautions (5.3)].
5.3 Monitoring Laboratory Tests
If assessment of plasma Factor VIII activity is needed, it is recommended to use a validated one-stage clotting assay [see Dosage and Administration (2)]. The ALTUVIIIO Factor VIII activity level is overestimated by the chromogenic assay and a specific ellagic acid based aPTT reagent in one-stage clotting assay by approximately 2.5-fold. If these assays are used, divide the result by 2.5 to approximate the patient's ALTUVIIIO Factor VIII activity level. Use of a reference laboratory is recommended when a qualified one-stage clotting assay or chromogenic assay is not available locally.
Monitor for the development of Factor VIII inhibitors. If bleeding is not controlled with ALTUVIIIO and the expected factor VIII activity plasma levels are not attained, perform an assay to determine if Factor VIII inhibitors are present (use Bethesda Units to titer inhibitors).
6. Adverse Reactions/Side Effects
The most common adverse reactions (>10% of subjects) reported in clinical trials were headache and arthralgia.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ALTUVIIIO has been evaluated in 159 previously treated patients (PTPs) (134 adults and 25 adolescents) with severe Hemophilia A (<1% endogenous FVIII activity or a genetic mutation consistent with severe Hemophilia A) who received at least one dose of ALTUVIIIO for either routine prophylaxis, on-demand treatment of bleeding episodes or perioperative management. A total of 152 (96%) subjects achieved at least 25 exposure days and 115 (72%) subjects achieved at least 50 exposure days with a median of 53.0 (range 2–63) for both exposure days and injections per subject. Overall exposure was monitored for a total of 151.5 subject-years. Adverse drug reactions (ADRs) (summarized in Table 3) were reported in 57 (36%) of the 159 subjects treated with routine prophylaxis or on-demand therapy. There were no age-specific differences in ADRs observed between adolescent and adult subjects. In the study, no inhibitors were detected and no ADRs of anaphylaxis were reported.
| MedDRA System Organ Class | Adverse Drug Reactions | Number of Subjects n (%) (N = 159) |
|---|---|---|
|
||
| Nervous system disorders | Headache* | 33 (21) |
| Musculoskeletal and connective tissue disorders | ||
| Arthralgia | 26 (16) | |
| Back pain | 9 (6) | |
In the ongoing pediatric study, the safety of ALTUVIIIO was evaluated in 67 male PTPs <12 years of age with severe hemophilia A who received at least one dose of ALTUVIIIO. At the time of the interim analysis, a total of 23 (34%) subjects achieved at least 25 exposure days with a median of 14.0 (range 1–46) for both exposure days and injections per subject.
Adverse drug reactions, headache, were reported in 1 (1%) of subjects. In the study, no inhibitors were detected and no ADRs of anaphylaxis were reported.
Thromboembolic events occurred in 1% (3/206) of subjects in the long term safety extension study; these three subjects had pre-existing risk factors.
8. Use In Specific Populations
8.4 Pediatric Use
Safety, efficacy, and pharmacokinetics (PK) have been evaluated in 92 previously treated, pediatric patients <18 years of age who received at least one dose of ALTUVIIIO as part of routine prophylaxis, treatment of bleeding episodes, or perioperative management. Adolescent subjects were enrolled in the adult and adolescent study and pediatric subjects <12 years of age were enrolled in an ongoing pediatric trial. Thirty-one subjects (33.7%) were <6 years of age, 36 (39.1%) subjects were 6 to <12 years of age, and 25 subjects (27.2%) were adolescents (12 to <18 years of age). Interim data from a pediatric study of the 67 subjects <12 years of age showed that no dosing adjustment was required [see Clinical Pharmacology (12)].
8.5 Geriatric Use
Clinical studies of ALTUVIIIO did not include sufficient numbers of subjects 65 years of age and older to determine whether or not they respond differently from younger subjects. However, clinical experience with other Factor VIII products has not identified differences between the elderly and younger patients.
11. Altuviiio Description
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] is a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution for intravenous injection. The product is supplied in single-dose vials containing nominal potencies of 250, 500, 750, 1000, 2000, 3000, or 4000 international units (IU). Each vial of ALTUVIIIO is labeled with the actual Factor VIII activity content in IU. The powder for injection is reconstituted with 3 mL sterile water for injection (sWFI) supplied in a sterile prefilled syringe. The reconstituted solution should be essentially free of particles. The final product contains the excipients: arginine hydrochloride (250 mM), calcium chloride dihydrate (5 mM), histidine (10 mM), polysorbate 80 (0.05% w/v), and sucrose (5% w/v).
The active ingredient in ALTUVIIIO is a fully recombinant fusion protein comprising a single chain B-domain deleted (BDD) analogue of human FVIII covalently fused to the Fc domain of human immunoglobulin G1 (IgG1), the FVIII-binding D'D3 domain of human von Willebrand factor (VWF), and 2 XTEN polypeptides. ALTUVIIIO contains 2829 amino acids with an apparent molecular weight of 312 kDa. ALTUVIIIO is synthesized as 2 polypeptide chains which are covalently linked by 2 Fc hinge disulfide bonds. The first FVIII-XTEN-Fc polypeptide chain contains the A1A2 domain of FVIII along with 5 amino acids from B-domain (1–745 amino acids) fused to the 288-XTEN polypeptide (in place of the natural FVIII B-domain), the A3C1C2 domain of FVIII (1649–2332), and the Fc domain of human IgG1. The second VWF-XTEN-a2-Fc polypeptide chain contains the D'D3 domain of VWF (1–477 amino acids) fused to the 144-XTEN polypeptide, a thrombin cleavable acidic region 2 sequence from FVIII and the Fc domain of human IgG1. The Fc domain includes the hinge, CH2, and CH3 domains of IgG1. The Fc, VWF, and XTEN portions of the molecule extend the half-life of ALTUVIIIO in plasma.
ALTUVIIIO is produced by recombinant DNA technology in a human embryonic kidney (HEK) cell line, which has been extensively characterized. ALTUVIIIO is manufactured without addition of human- or animal-derived components and purified by a combination of multiple chromatography steps, a detergent viral inactivation step, a nano filtration step for viral clearance, and ultrafiltration steps.
12. Altuviiio - Clinical Pharmacology
12.1 Mechanism of Action
ALTUVIIIO [antihemophilic factor (recombinant), Fc-VWF-XTEN fusion protein-ehtl] temporarily replaces the missing coagulation factor VIII needed for effective hemostasis. ALTUVIIIO has demonstrated 3- to 4-fold prolonged half-life relative to other standard and extended half-life FVIII products.
12.2 Pharmacodynamics
Hemophilia A is a bleeding disorder characterized by a deficiency of functional coagulation factor VIII (FVIII), which leads to a prolonged clotting time in the activated partial thromboplastin time (aPTT)-based one-stage clotting assay. Administration of ALTUVIIIO increases plasma levels of FVIII, temporarily correcting the coagulation defect in hemophilia A patients.
Based on FVIII pharmacokinetic/pharmacodynamic analyses, the risk of bleeding is negatively correlated with FVIII activity. Once weekly 50 IU/kg ALTUVIIIO provided factor VIII activity levels that were associated with a low bleed risk.
12.3 Pharmacokinetics
The PK of ALTUVIIIO were evaluated in prospective, open-label clinical studies, enrolling 159 adults and adolescents, and 67 children <12 years old, respectively, receiving weekly IV injection of 50 IU/kg. Among children <12 years old, 32 subjects had ALTUVIIIO single dose PK profiles available.
PK parameters following a single dose of ALTUVIIIO are presented in Table 4. The PK parameters were based on plasma FVIII activity measured by the aPTT-based one-stage clotting assay. After a single dose of 50 IU/kg, ALTUVIIIO exhibited high sustained FVIII activity with prolonged half-life across age cohorts. There was a trend of increasing area under the curve (AUC), and decreasing clearance, with increasing age in the pediatric cohorts. The PK profile at steady state (Week 26) was comparable with the PK profile obtained after the first dose.
| PK Parameters (mean SD) | Pediatric Study | Pediatric Study | Adult and Adolescent Study | Adult and Adolescent Study |
|---|---|---|---|---|
| 1 to <6 Years N = 14 | 6 to <12 Years N = 18 | 12 to <18 years N = 25 | Adults N = 134 |
|
| AUC0–tau = area under the activity-time curve over the dosing interval, CL = clearance, MRT = mean residence time, SD = standard deviation, t1/2z = terminal half-life, Vss = volume of distribution at steady state. | ||||
|
||||
| AUC (IU×h/dL) | 6710 (1190) | 7190 (1450) | 8350 (1550) | 9850 (2010) * |
| t1/2 (h) | 39.9 (5.71) | 42.4 (3.70) | 44.6 (4.99) | 48.2 (9.31) |
| CL (mL/h/kg) | 0.740 (0.128) | 0.681 (0.139) | 0.582 (0.115) | 0.493 (0.121) * |
| Vss (mL/kg) | 38.0 (7.19) | 38.1 (6.80) | 34.9 (7.38) | 31.0 (7.32) * |
| MRT (hr) | 51.9 (9.06) | 56.3 (5.10) | 60.0 (5.54) | 63.9 (10.2) * |
ALTUVIIIO at steady state maintained normal to near normal (>40 IU/dL) FVIII activity for a mean (SD) of 4.1 (0.7) days with once weekly prophylaxis in adults. The FVIII activity over 10 IU/dL was maintained in 83.5% of adults and adolescent subjects throughout the study. In children <12 years ALTUVIIIO maintained normal to near normal (>40 IU/dL) FVIII activity for 2 to 3 days and >10 IU/dL FVIII activity for approximately 6 to 7 days. The majority of children <12 years maintained FVIII activity in mild hemophilia range (>5 IU/dL) 7 days after the dosing (see Table 5).
| PK Parameters Mean (SD) | Pediatric Study* | Pediatric Study* | Adult and Adolescent Study* | Adult and Adolescent Study* |
|---|---|---|---|---|
| 1 to <6 years N = 20 | 6 to <12 years N = 35 | 12 to <18 years N = 24 | Adults N = 124 |
|
| Peak = 15 min post dose at steady state, IR = incremental recovery, Trough – predose FVIII activity value at steady state, SD = standard deviation. | ||||
|
||||
| Peak (IU/dL) | 113 (26.2) | 121 (25.31) | 124 (31.2) | 150 (35.0) |
| IR (kg×IU/dL/IU) | 2.10 (0.53) | 2.17 (0.46) | 2.25 (0.61) | 2.64 (0.61) |
| Time to 40 IU/dL (h) † | 59.2 (7.68) | 72.2 (11.2) | 81.7 (13.1) | 97.0 (20.2) |
| Time to 20 IU/dL (h) † | 99.3 (9.15) | 117 (13.9) | 130 (15.7) | 150 (27.7) |
| Time to 10 IU/dL (h) † | 139 (11.7) | 163 (17.1) | 179 (21.4) | 200 (35.4) |
| Trough (IU/dL) | 6.75 (2.29) | 9.77 (3.64) | 9.23 (4.77) | 18.0 (16.6) |
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, or of other Factor VIII products.
All subjects were monitored for neutralizing antibodies (inhibitors) to Factor VIII in the clinical program. No subjects developed neutralizing antibodies to Factor VIII.
During treatment (up to 49.64 weeks) in the clinical studies, 4/277 (2.2%) of ALTUVIIIO patients developed anti-drug antibodies.
No impact of ADAs on the FVIII activity-time profiles and PK exposure parameters was observed.
No impact of ADAs with respect to bleeding episodes and in the pharmacodynamic response was noted.
14. Clinical Studies
The safety, efficacy, and pharmacokinetics of ALTUVIIIO were evaluated in two multicenter, prospective, open-label clinical studies (one study in adults and adolescents ≥12 years of age and one pediatric study in children <12 years of age) in previously treated patients (PTPs) with severe hemophilia A (<1% endogenous Factor VIII activity or a documented genetic mutation consistent with severe hemophilia A).
All studies evaluated the efficacy of routine prophylaxis with a weekly dose of 50 IU/kg and determined hemostatic efficacy in the treatment of bleeding episodes and during perioperative management in subjects undergoing major or minor surgical procedures.
The completed adult and adolescent study enrolled a total of 159 PTPs (158 male and 1 female subjects) with severe hemophilia A. Subjects were aged 12 to 72 years and included 25 adolescent subjects aged 12 to 17 years. All 159 enrolled subjects received at least one dose of ALTUVIIIO and were evaluable for efficacy. A total of 149 subjects (93.7%) completed the study.
The ongoing pediatric study enrolled 67 male PTPs <12 years of age with severe hemophilia A (31 subjects were 1 to 5 years of age and 36 were 6 to 11 years of age) at data cut-off. Of the 67 enrolled subjects, all received at least 1 dose of ALTUVIIIO.
17. Patient Counseling Information
Advise the patients to:
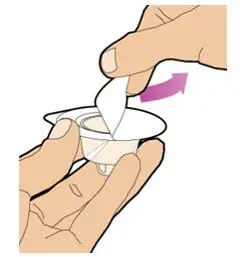
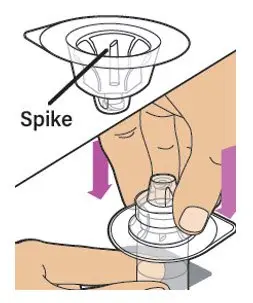
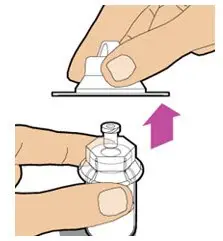
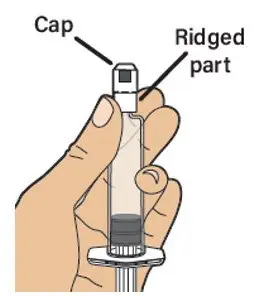
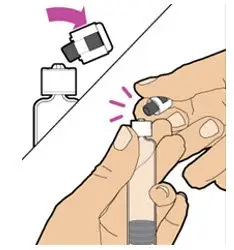
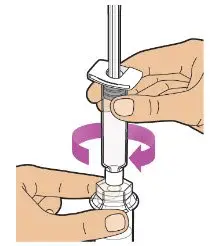
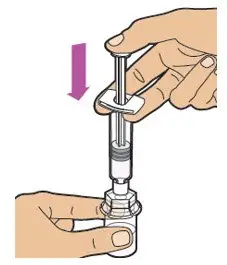
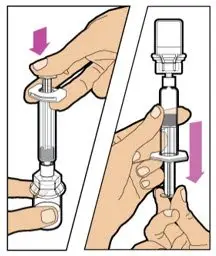
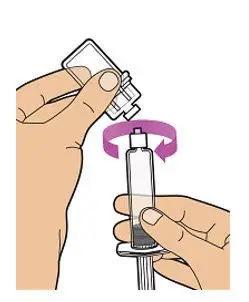
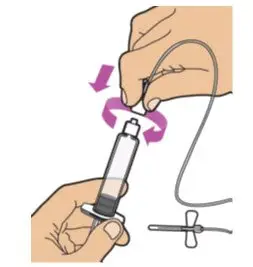
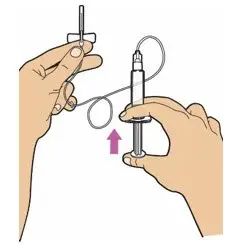
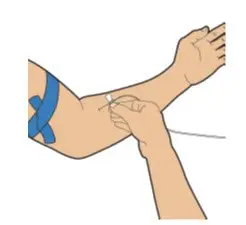
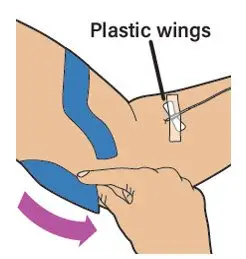
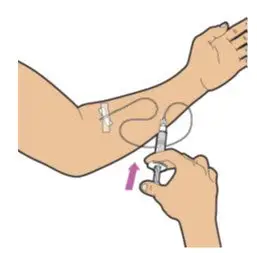
- Read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Call their healthcare provider or go to the emergency department right away if a hypersensitivity reaction occurs. Early signs of hypersensitivity reactions may include rash, hives, itching, facial swelling, tightness of the chest, and wheezing.
- Contact their healthcare provider or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to Factor VIII therapy because this may be a sign of inhibitor development.
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| ALTUVIIIO
antihemophilic factor (recombinant), fc-vwf-xten fusion protein-ehtl kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bioverativ Therapeutics Inc. (070517011) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PPD Development Ireland Ltd. | 985036175 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biopharma Product Testing Munich GmbH | 313046917 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Lancaster Laboratories, Inc | 069777290 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Biogen U.S. Corporation | 078734950 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , MANUFACTURE(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Rechon Life Science AB | 775207769 | PACK(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , LABEL(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | PACK(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , LABEL(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma Fertigung GmbH & Co. KG (Langenargen Eisenbahnstrasse) | 344217323 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , MANUFACTURE(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Schuetzenstrasse) | 316126754 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vetter Pharma Fertigung GmbH & Co. KG (Ravensburg Mooswiesen) | 312670654 | ANALYSIS(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) , PACK(71104-978, 71104-979, 71104-981, 71104-982, 71104-983, 71104-984) | |




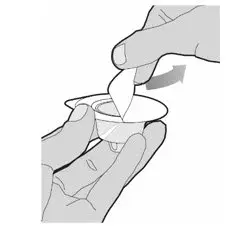
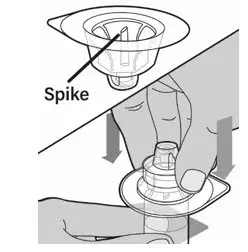
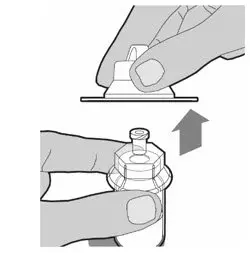
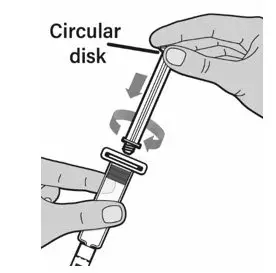


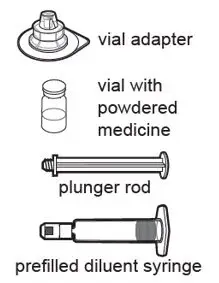





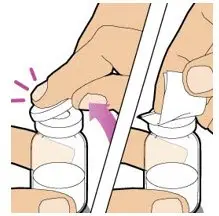
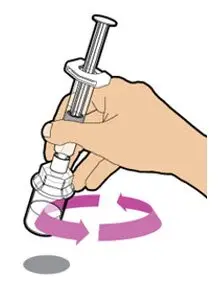
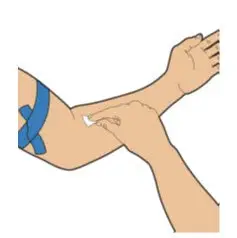
 After cleaning,
After cleaning,