Drug Detail:Alunbrig (Brigatinib)
Drug Class: Multikinase inhibitors
Highlights of Prescribing Information
ALUNBRIG® (brigatinib) tablets, for oral use
Initial U.S. Approval: 2017
Recent Major Changes
| Dosage and Administration Dosage Modifications for Adverse Reactions (2.3) | 2/2022 |
| Warnings and Precautions Hepatic Enzymes Elevation (5.7) | 2/2022 |
| Warnings and Precautions, Photosensitivity (5.9) | 9/2021 |
Indications and Usage for Alunbrig
ALUNBRIG is a kinase inhibitor indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) as detected by an FDA-approved test. (1, 2.1)
Alunbrig Dosage and Administration
90 mg orally once daily for the first 7 days; then increase to 180 mg orally once daily. May be taken with or without food. (2.2)
Dosage Forms and Strengths
Tablets: 180 mg, 90 mg, or 30 mg. (3)
Contraindications
None. (4)
Warnings and Precautions
- Interstitial Lung Disease (ILD)/Pneumonitis: Monitor for new or worsening respiratory symptoms, particularly during the first week of treatment. Withhold ALUNBRIG for new or worsening respiratory symptoms and promptly evaluate for ILD/pneumonitis. Upon recovery, either dose reduce or permanently discontinue ALUNBRIG. (2.3, 5.1)
- Hypertension: Monitor blood pressure after 2 weeks and then at least monthly during treatment. For severe hypertension, withhold ALUNBRIG, then dose reduce or permanently discontinue. (2.3, 5.2)
- Bradycardia: Monitor heart rate and blood pressure regularly during treatment. If symptomatic, withhold ALUNBRIG, then dose reduce or permanently discontinue. (2.3, 5.3)
- Visual Disturbance: Advise patients to report visual symptoms. Withhold ALUNBRIG and obtain ophthalmologic evaluation, then dose reduce or permanently discontinue ALUNBRIG. (2.3, 5.4)
- Creatine Phosphokinase (CPK) Elevation: Monitor CPK levels regularly during treatment. Based on the severity and with muscle pain or weakness, withhold ALUNBRIG, then resume or reduce dose. (2.3, 5.5)
- Pancreatic Enzymes Elevation: Monitor lipase and amylase levels regularly during treatment. Based on the severity, withhold ALUNBRIG, then resume or reduce dose. (2.3, 5.6)
- Hepatotoxicity: Monitor alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin levels regularly during treatment. Based on severity, withhold dose, then resume at lower level. (2.3, 5.7)
- Hyperglycemia: Assess fasting serum glucose prior to starting ALUNBRIG and regularly during treatment. If not adequately controlled with optimal medical management, withhold ALUNBRIG, then consider dose reduction or permanently discontinue, based on severity. (2.3, 5.8)
- Photosensitivity: Advise patients to limit sun exposure. Based on severity withhold ALUNBRIG, then resume at the same dose, reduce the dose, or permanently discontinue. (2.3, 5.9)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.10, 8.1, 8.3)
Adverse Reactions/Side Effects
The most common adverse reactions (≥25%) with ALUNBRIG were diarrhea, fatigue, nausea, rash, cough, myalgia, headache, hypertension, vomiting, and dyspnea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-844-217-6468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A Inhibitors: Avoid coadministration of ALUNBRIG with strong or moderate CYP3A inhibitors. If coadministration of a strong or moderate CYP3A inhibitor is unavoidable, reduce the dose of ALUNBRIG. (2.4, 7.1)
- CYP3A Inducers: Avoid coadministration of ALUNBRIG with strong or moderate CYP3A inducers. If coadministration of a moderate CYP3A inducer is unavoidable, increase the dose of ALUNBRIG. (2.5, 7.1)
Use In Specific Populations
- Hepatic Impairment: Reduce the dose of ALUNBRIG for patients with severe hepatic impairment. (2.6, 8.6)
- Renal Impairment: Reduce the dose of ALUNBRIG for patients with severe renal impairment. (2.7, 8.7)
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2022
Full Prescribing Information
1. Indications and Usage for Alunbrig
ALUNBRIG is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) as detected by an FDA-approved test [see Dosage and Administration (2.1)].
2. Alunbrig Dosage and Administration
2.1 Patient Selection
Select patients for the treatment of metastatic NSCLC with ALUNBRIG based on the presence of ALK positivity in tumor specimens [see Clinical Studies (14)].
Information on FDA-approved tests for the detection of ALK rearrangements in NSCLC is available at http://www.fda.gov/CompanionDiagnostics.
2.2 Recommended Dosage
The recommended dosage for ALUNBRIG is:
- 90 mg orally once daily for the first 7 days; then increase the dose to 180 mg orally once daily.
Administer ALUNBRIG until disease progression or unacceptable toxicity.
If ALUNBRIG is interrupted for 14 days or longer for reasons other than adverse reactions, resume treatment at 90 mg once daily for 7 days before increasing to the previously tolerated dose.
ALUNBRIG may be taken with or without food. Instruct patients to swallow tablets whole. Do not crush or chew tablets.
If a dose of ALUNBRIG is missed or vomiting occurs after taking a dose, do not administer an additional dose and take the next dose of ALUNBRIG at the scheduled time.
2.3 Dosage Modifications for Adverse Reactions
ALUNBRIG dosage reductions for adverse reactions are summarized in Table 1.
| Dosage Reduction | |||
|---|---|---|---|
| Dosage | First | Second | Third |
|
|||
| 90 mg once daily | 60 mg once daily | permanently discontinue | N/A* |
| 180 mg once daily | 120 mg once daily | 90 mg once daily | 60 mg once daily |
Once reduced for adverse reactions, do not subsequently increase the dosage of ALUNBRIG. Permanently discontinue ALUNBRIG if patients are unable to tolerate the 60 mg once daily dose.
Recommendations for dosage modifications of ALUNBRIG for the management of adverse reactions are provided in Table 2.
| Adverse Reaction | Severity* | Dosage Modifications |
|---|---|---|
| bpm = beats per minute; DBP = diastolic blood pressure; HR = heart rate; SBP = systolic blood pressure; ULN = upper limit of normal | ||
|
||
| Interstitial Lung Disease (ILD) /Pneumonitis [see Warnings and Precautions (5.1)] | Grade 1 |
|
| Grade 2 |
|
|
| Grade 3 or 4 | Permanently discontinue ALUNBRIG for ILD/pneumonitis. | |
| Hypertension [see Warnings and Precautions (5.2)] | Grade 3 hypertension (SBP greater than or equal to 160 mmHg or DBP greater than or equal to 100 mmHg, medical intervention indicated, more than one antihypertensive drug, or more intensive therapy than previously used indicated) |
|
| Grade 4 hypertension (life-threatening consequences, urgent intervention indicated) |
|
|
| Bradycardia (HR less than 60 bpm) [see Warnings and Precautions (5.3)] | Symptomatic bradycardia |
|
| Bradycardia with life-threatening consequences, urgent intervention indicated |
|
|
| Visual Disturbance [see Warnings and Precautions (5.4)] | Grade 2 or 3 visual disturbance | Withhold ALUNBRIG until recovery to Grade 1 or baseline, then resume at the next lower dose (Table 1). |
| Grade 4 visual disturbance | Permanently discontinue ALUNBRIG. | |
| Creatine Phosphokinase (CPK) Elevation [see Warnings and Precautions (5.5)] | Grade 3 or 4 CPK elevation (greater than 5× ULN) with Grade 2 or higher muscle pain or weakness |
|
| Lipase/Amylase Elevation [see Warnings and Precautions (5.6)] | Grade 3 lipase or amylase elevation (greater than 2× ULN) |
|
| Grade 4 lipase or amylase elevation (greater than 5× ULN) | Withhold ALUNBRIG until recovery to Grade 1 or less (less than or equal to 1.5× ULN) or to baseline, then resume ALUNBRIG at next lower dose (Table 1). | |
| Hepatotoxicity (Elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST)) [see Warnings and Precautions (5.7)] | Grade 3 or 4 elevation (greater than 5 × ULN) of either ALT or AST with bilirubin less than or equal to 2 × ULN | Withhold ALUNBRIG until recovery to Grade 1 or less (less than or equal to 3× ULN) or to baseline, then resume ALUNBRIG at next lower dose (Table 1). |
| Grade 2 to 4 elevation (greater than 3 × ULN) of ALT or AST with concurrent total bilirubin elevation greater than 2 × ULN in the absence of cholestasis or hemolysis | Permanently discontinue ALUNBRIG. | |
| Hyperglycemia [see Warnings and Precautions (5.8)] | Grade 3 (greater than 250 mg/dL or 13.9 mmol/L) or 4 | If adequate hyperglycemic control cannot be achieved with optimal medical management, withhold ALUNBRIG until adequate hyperglycemic control is achieved and resume at the next lower dose (Table 1) or permanently discontinue ALUNBRIG. |
| Other | Grade 3 |
|
| Grade 4 |
|
|
2.4 Dosage Modifications for Strong or Moderate CYP3A Inhibitors
Avoid coadministration of strong or moderate CYP3A inhibitors during treatment with ALUNBRIG [see Drug Interactions (7.1), Clinical Pharmacology (12.3)]. If coadministration of a strong CYP3A inhibitor cannot be avoided, reduce the ALUNBRIG once daily dose by approximately 50% (i.e., from 180 mg to 90 mg, or from 90 mg to 60 mg). If coadministration of a moderate CYP3A inhibitor cannot be avoided, reduce the ALUNBRIG once daily dose by approximately 40% (i.e., from 180 mg to 120 mg, 120 mg to 90 mg, or from 90 mg to 60 mg). After discontinuation of a strong or moderate CYP3A inhibitor, resume the ALUNBRIG dose that was tolerated prior to initiating the CYP3A inhibitor.
2.5 Dosage Modifications for Moderate CYP3A Inducers
Avoid coadministration of moderate CYP3A inducers during treatment with ALUNBRIG [see Drug Interactions (7.1), Clinical Pharmacology (12.3)]. If coadministration of a moderate CYP3A inducer cannot be avoided, increase the ALUNBRIG once daily dose in 30 mg increments after 7 days of treatment with the current ALUNBRIG dose as tolerated, up to a maximum of twice the ALUNBRIG dose that was tolerated prior to initiating the moderate CYP3A inducer.
After discontinuation of a moderate CYP3A inducer, resume the ALUNBRIG dose that was tolerated prior to initiating the moderate CYP3A inducer.
2.6 Dosage Modifications for Patients with Severe Hepatic Impairment
Reduce the ALUNBRIG once daily dose by approximately 40% (i.e., from 180 mg to 120 mg, 120 mg to 90 mg, or from 90 mg to 60 mg) for patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
2.7 Dosage Modifications for Patients with Severe Renal Impairment
Reduce the ALUNBRIG once daily dose by approximately 50% (i.e., from 180 mg to 90 mg, or from 90 mg to 60 mg) for patients with severe renal impairment [creatinine clearance (CLcr) 15 to 29 mL/min by Cockcroft-Gault] [see Use in Specific Populations (8.7), Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
- 180 mg: oval, white to off-white film-coated tablets with "U13" debossed on one side and plain on the other side
- 90 mg: oval, white to off-white film-coated tablets with "U7" debossed on one side and plain on the other side
- 30 mg: round, white to off-white film-coated tablets with "U3" debossed on one side and plain on the other side
5. Warnings and Precautions
5.1 Interstitial Lung Disease (ILD)/Pneumonitis
Severe, life-threatening, and fatal pulmonary adverse reactions consistent with interstitial lung disease (ILD)/pneumonitis have occurred with ALUNBRIG.
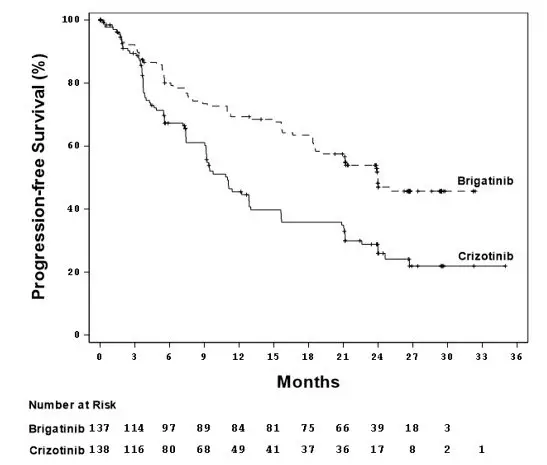
In Trial ALTA 1L, ILD/pneumonitis occurred in 5.1% of patients receiving ALUNBRIG. ILD/pneumonitis occurred within 8 days of initiation of ALUNBRIG in 2.9% of patients, with Grade 3 to 4 reactions occurring in 2.2% of patients.
In Trial ALTA, ILD/pneumonitis occurred in 3.7% of patients in the 90 mg group (90 mg once daily) and 9.1% of patients in the 90→180 mg group (180 mg once daily with 7-day lead-in at 90 mg once daily). Adverse reactions consistent with possible ILD/pneumonitis occurred within 9 days of initiation of ALUNBRIG (median onset was 2 days) in 6.4% of patients, with Grade 3 to 4 reactions occurring in 2.7%.
Monitor for new or worsening respiratory symptoms (e.g., dyspnea, cough, etc.), particularly during the first week of initiating ALUNBRIG. Withhold ALUNBRIG in any patient with new or worsening respiratory symptoms, and promptly evaluate for ILD/pneumonitis or other causes of respiratory symptoms (e.g., pulmonary embolism, tumor progression, and infectious pneumonia). For Grade 1 or 2 ILD/pneumonitis, either resume ALUNBRIG with dose reduction according to Table 1 after recovery to baseline or permanently discontinue ALUNBRIG. Permanently discontinue ALUNBRIG for Grade 3 or 4 ILD/pneumonitis or recurrence of Grade 1 or 2 ILD/pneumonitis [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.2 Hypertension
In ALTA 1L, hypertension was reported in 32% of patients receiving ALUNBRIG; Grade 3 hypertension occurred in 13% of patients.
In ALTA, hypertension was reported in 11% of patients in the 90 mg group who received ALUNBRIG and 21% of patients in the 90→180 mg group. Grade 3 hypertension occurred in 5.9% of patients overall.
Control blood pressure prior to treatment with ALUNBRIG. Monitor blood pressure after 2 weeks and at least monthly thereafter during treatment with ALUNBRIG. Withhold ALUNBRIG for Grade 3 hypertension despite optimal antihypertensive therapy. Upon resolution or improvement to Grade 1, resume ALUNBRIG at the same dose. Consider permanent discontinuation of treatment with ALUNBRIG for Grade 4 hypertension or recurrence of Grade 3 hypertension [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
Use caution when administering ALUNBRIG in combination with antihypertensive agents that cause bradycardia [see Warnings and Precautions (5.3)].
5.3 Bradycardia
In ALTA 1L, heart rates less than 50 beats per minute (bpm) occurred in 8.1% of patients receiving ALUNBRIG. Grade 3 bradycardia occurred in 1 patient (0.7%).
In ALTA, heart rates less than 50 beats per minute (bpm) occurred in 5.7% of patients in the 90 mg group and 7.6% of patients in the 90→180 mg group. Grade 2 bradycardia occurred in 1 (0.9%) patient in the 90 mg group.
Monitor heart rate and blood pressure during treatment with ALUNBRIG. Monitor patients more frequently if concomitant use of drug known to cause bradycardia cannot be avoided [see Warnings and Precautions (5.2)].
For symptomatic bradycardia, withhold ALUNBRIG and review concomitant medications for those known to cause bradycardia. If a concomitant medication known to cause bradycardia is identified and discontinued or dose adjusted, resume ALUNBRIG at the same dose following resolution of symptomatic bradycardia; otherwise, reduce the dose of ALUNBRIG following resolution of symptomatic bradycardia. Discontinue ALUNBRIG for life-threatening bradycardia if no contributing concomitant medication is identified [see Dosage and Administration (2.3)].
5.4 Visual Disturbance
In ALTA 1L, Grade 1 or 2 adverse reactions leading to visual disturbance including blurred vision, photophobia, photopsia, and reduced visual acuity were reported in 7.4% of patients receiving ALUNBRIG.
In ALTA, adverse reactions leading to visual disturbance including blurred vision, diplopia, and reduced visual acuity, were reported in 7.3% of patients receiving ALUNBRIG in the 90 mg group and 10% of patients in the 90→180 mg group. Grade 3 macular edema and cataract occurred in 1 patient each in the 90→180 mg group.
Advise patients to report any visual symptoms. Withhold ALUNBRIG and obtain an ophthalmologic evaluation in patients with new or worsening visual symptoms of Grade 2 or greater severity. Upon recovery of Grade 2 or Grade 3 visual disturbances to Grade 1 severity or baseline, resume ALUNBRIG at a reduced dose. Permanently discontinue treatment with ALUNBRIG for Grade 4 visual disturbances [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.5 Creatine Phosphokinase (CPK) Elevation
In ALTA 1L, creatine phosphokinase (CPK) elevation occurred in 81% of patients who received ALUNBRIG. The incidence of Grade 3 or 4 CPK elevation was 24%. Dose reduction for CPK elevation occurred in 15% of patients.
In ALTA, CPK elevation occurred in 27% of patients receiving ALUNBRIG in the 90 mg group and 48% of patients in the 90 mg→180 mg group. The incidence of Grade 3 to 4 CPK elevation was 2.8% in the 90 mg group and 12% in the 90→180 mg group.
Dose reduction for CPK elevation occurred in 1.8% of patients in the 90 mg group and 4.5% in the 90→180 mg group.
Advise patients to report any unexplained muscle pain, tenderness, or weakness. Monitor CPK levels during ALUNBRIG treatment. Withhold ALUNBRIG for Grade 3 or 4 CPK elevation with Grade 2 or higher muscle pain or weakness. Upon resolution or recovery to Grade 1 CPK elevation or baseline, resume ALUNBRIG at the same dose or at a reduced dose as described in Table 2 [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.6 Pancreatic Enzymes Elevation
In ALTA 1L, amylase elevation occurred in 52% of patients and Grade 3 or 4 amylase elevation occurred in 6.8% of patients. Lipase elevations occurred in 59% of patients and Grade 3 or 4 lipase elevation occurred in 17% of patients.
In ALTA, amylase elevation occurred in 27% of patients in the 90 mg group and 39% of patients in the 90→180 mg group. Lipase elevations occurred in 21% of patients in the 90 mg group and 45% of patients in the 90→180 mg group. Grade 3 or 4 amylase elevation occurred in 3.7% of patients in the 90 mg group and 2.7% of patients in the 90→180 mg group. Grade 3 or 4 lipase elevation occurred in 4.6% of patients in the 90 mg group and 5.5% of patients in the 90→180 mg group.
Monitor lipase and amylase during treatment with ALUNBRIG. Withhold ALUNBRIG for Grade 3 or 4 pancreatic enzyme elevation. Upon resolution or recovery to Grade 1 or baseline, resume ALUNBRIG at the same dose or at a reduced dose as described in Table 2 [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.7 Hepatotoxicity
In ALTA 1L, aspartate aminotransferase (AST) elevations occurred in 72% of patients and Grade 3 or 4 AST elevations occurred in 4.5% of patients. Alanine aminotransferase (ALT) elevations occurred in 52% of patients and Grade 3 or 4 ALT elevations occurred in 5.2% of patients. One patient (0.7%) had a serious adverse reaction of hepatocellular injury.
In ALTA, AST elevations occurred in 38% of patients in the 90 mg group and 65% of patients in the 90→180 mg group. ALT elevations occurred in 34% of patients in the 90 mg group and 40% of patients in the 90→180 mg group. Grade 3 or 4 AST elevations occurred in 0.9% of patients in the 90 mg group and did not occur in any patients in the 90→180 mg group. Grade 3 or 4 ALT elevations did not occur in any patients in the 90 mg group and in 2.7% of patients in the 90→180 mg group.
Monitor AST, ALT and total bilirubin during treatment with ALUNBRIG, especially during first 3 months. Withhold ALUNBRIG for Grade 3 or 4 hepatic enzyme elevation with bilirubin less than or equal to 2 × ULN. Upon resolution or recovery to Grade 1 or less (less than or equal to 3 × ULN) or to baseline, resume ALUNBRIG at a next lower dose as described in Table 2. Permanently discontinue ALUNBRIG for Grade 2 to 4 hepatic enzyme elevation with concurrent total bilirubin elevation greater than 2 times the ULN in the absence of cholestasis or hemolysis [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.8 Hyperglycemia
In ALTA 1L, 56% of patients who received ALUNBRIG experienced new or worsening hyperglycemia. Grade 3 hyperglycemia, based on laboratory assessment of serum fasting glucose levels, occurred in 7.5% of patients.
In ALTA, 43% of patients who received ALUNBRIG experienced new or worsening hyperglycemia. Grade 3 hyperglycemia, based on laboratory assessment of serum fasting glucose levels, occurred in 3.7% of patients. Two of 20 (10%) patients with diabetes or glucose intolerance at baseline required initiation of insulin while receiving ALUNBRIG.
Assess fasting serum glucose prior to initiation of ALUNBRIG and monitor periodically thereafter. Initiate or optimize antihyperglycemic medications as needed. If adequate hyperglycemic control cannot be achieved with optimal medical management, withhold ALUNBRIG until adequate hyperglycemic control is achieved and consider reducing the dose of ALUNBRIG as described in Table 1 or permanently discontinuing ALUNBRIG [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.9 Photosensitivity
In ALTA 1L, photosensitivity occurred in 3.7% of patients who received ALUNBRIG, with 0.7% Grade 3 to 4.
In ALTA, 0.9% of patients who received ALUNBRIG in the 90 mg group experienced photosensitivity and 0.9% of patients in the 90 mg→180 mg group. Grade 3 to 4 photosensitivity was not reported in patients in the 90 mg group or in the 90→180 mg group.
Advise patients to limit sun exposure while taking brigatinib, and for at least 5 days after discontinuation of treatment. Advise patients, when outdoors to wear a hat and protective clothing, and use a broad-spectrum Ultraviolet A (UVA)/ Ultraviolet B (UVB) sunscreen and lip balm (SPF ≥30) to help protect against sunburn. Based on the severity withhold ALUNBRIG, then resume at the same dose, or reduce the dose, or permanently discontinue as described in Table 2 [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
5.10 Embryo-Fetal Toxicity
Based on its mechanism of action and findings in animals, ALUNBRIG can cause fetal harm when administered to pregnant women. There are no clinical data on the use of ALUNBRIG in pregnant women. Administration of brigatinib to pregnant rats during the period of organogenesis resulted in dose-related skeletal anomalies at doses as low as 12.5 mg/kg/day (approximately 0.7 times the human exposure by AUC at 180 mg once daily), as well as increased post-implantation loss, malformations, and decreased fetal body weight at doses of 25 mg/kg/day (approximately 1.26 times the human exposure at 180 mg once daily) or higher.
Advise women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ALUNBRIG and for at least 4 months following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment and for at least 3 months after the last dose of ALUNBRIG [see Use in Specific Populations (8.1, 8.3), Clinical Pharmacology (12.1)].
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the prescribing information:
- Interstitial Lung Disease (ILD)/Pneumonitis [see Warnings and Precautions (5.1)]
- Hypertension [see Warnings and Precautions (5.2)]
- Bradycardia [see Warnings and Precautions (5.3)]
- Visual Disturbance [see Warnings and Precautions (5.4)]
- Creatine Phosphokinase (CPK) Elevation [see Warnings and Precautions (5.5)]
- Pancreatic Enzymes Elevation [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
- Hyperglycemia [see Warnings and Precautions (5.8)]
- Photosensitivity [see Warnings and Precautions (5.9)]
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
The safety and effectiveness of ALUNBRIG in pediatric patients have not been established.
8.5 Geriatric Use
Of the 359 patients enrolled in the ALTA 1L ALUNBRIG arm and in ALTA, 26.7% were 65 and older and 7.5% were 75 and older. No overall differences in safety or effectiveness were observed between patients ≥65 years and younger patients.
8.6 Hepatic Impairment
No dose adjustment is recommended for patients with mild hepatic impairment (Child-Pugh A) or moderate hepatic impairment (Child-Pugh B). Reduce the dose of ALUNBRIG for patients with severe hepatic impairment (Child-Pugh C) [see Dosage and Administration (2.6), Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dose adjustment is recommended for patients with mild or moderate renal impairment [creatinine clearance (CLcr) 30 to 89 mL/min by Cockcroft-Gault]. Reduce the dose of ALUNBRIG for patients with severe renal impairment (CLcr 15 to 29 mL/min) [see Dosage and Administration (2.7), Clinical Pharmacology (12.3)].
11. Alunbrig Description
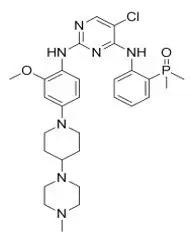
Brigatinib is a kinase inhibitor. The chemical name for brigatinib is 5-chloro-N4-[2-(dimethylphosphoryl)phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]phenyl}pyrimidine-2,4-diamine. The molecular formula is C29H39ClN7O2P which corresponds to a formula weight of 584.10 g/mol. Brigatinib has no chiral centers. The chemical structure is shown below:
Brigatinib is an off-white to beige/tan solid. The pKas were determined to be: 1.73 ± 0.02 (base), 3.65 ± 0.01 (base), 4.72 ± 0.01 (base), and 8.04 ± 0.01 (base).
ALUNBRIG is supplied for oral use as film-coated tablets containing 180 mg, 90 mg or 30 mg of brigatinib and the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate (Type A), magnesium stearate, and hydrophobic colloidal silica. The tablet coating consists of talc, polyethylene glycol, polyvinyl alcohol, and titanium dioxide.
12. Alunbrig - Clinical Pharmacology
12.1 Mechanism of Action
Brigatinib is a tyrosine kinase inhibitor (TKI) with in vitro activity at clinically achievable concentrations against multiple kinases including ALK, ROS1, insulin-like growth factor-1 receptor (IGF-1R), and FLT-3 as well as EGFR deletion and point mutations. Brigatinib inhibited autophosphorylation of ALK and ALK-mediated phosphorylation of the downstream signaling proteins STAT3, AKT, ERK1/2, and S6 in in vitro and in vivo assays. Brigatinib also inhibited the in vitro proliferation of cell lines expressing EML4-ALK and NPM-ALK fusion proteins and demonstrated dose-dependent inhibition of EML4-ALK-positive NSCLC xenograft growth in mice.
At clinically achievable concentrations (≤500 nM), brigatinib inhibited the in vitro viability of cells expressing EML4-ALK and 17 mutant forms associated with resistance to ALK inhibitors including crizotinib, as well as EGFR-Del (E746-A750), ROS1-L2026M, FLT3-F691L, and FLT3-D835Y. Brigatinib exhibited in vivo antitumor activity against 4 mutant forms of EML4-ALK, including G1202R and L1196M mutants identified in NSCLC tumors in patients who have progressed on crizotinib. Brigatinib also reduced tumor burden and prolonged survival in mice implanted intracranially with an ALK-driven tumor cell line.
12.2 Pharmacodynamics
Brigatinib exposure-response relationships and the time course of the pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The geometric mean (CV%) steady-state maximum concentration (Cmax) of brigatinib at ALUNBRIG doses of 90 mg and 180 mg once daily was 552 (49%) ng/mL and 1452 (60%) ng/mL, respectively, and the corresponding area under the concentration-time curve (AUC0-Tau) was 8165 (45%) ng∙h/mL and 20276 (62%) ng∙h/mL. After a single dose and multiple dosing of ALUNBRIG, systemic exposure of brigatinib was dose proportional over the dose range of 60 mg (0.3 times the recommended dose of 180 mg) to 240 mg (1.3 times the recommended dose of 180 mg) once daily. The mean accumulation ratio after repeat dosing was 1.9 to 2.4.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been performed with brigatinib.
Treatment with brigatinib resulted in chromosomal damage in an in vivo mammalian erythrocyte micronucleus in the rat, but was not mutagenic in the Ames or in vitro mammalian chromosome aberration tests.
Dedicated animal fertility studies were not conducted with brigatinib. Testicular toxicity was observed in repeat-dose animal studies at doses resulting in exposure as low as 0.2 times the exposure in patients at the 180 mg dose. In rats, findings included lower weight of testes, seminal vesicles and prostate gland, and testicular tubular degeneration; these effects were not reversible during the 2 month recovery period. In monkeys, findings included reduced size of testes along with microscopic evidence of hypospermatogenesis; these effects were reversible during the recovery period.
16. How is Alunbrig supplied
180 mg tablets: oval, white to off-white film-coated tablets with "U13" debossed on one side and plain on the other side; available in:
| Bottle of 23 tablets | NDC 63020-180-23 |
| Bottle of 30 tablets | NDC 63020-180-30 |
90 mg tablets: oval, white to off-white film-coated tablets with "U7" debossed on one side and plain on the other side; available in:
| Bottle of 7 tablets | NDC 63020-090-07 |
| Bottle of 30 tablets | NDC 63020-090-30 |
30 mg tablets: round, white to off-white film-coated tablets with "U3" debossed on one side and plain on the other side; available in:
| Bottle of 30 tablets | NDC 63020-113-30 |
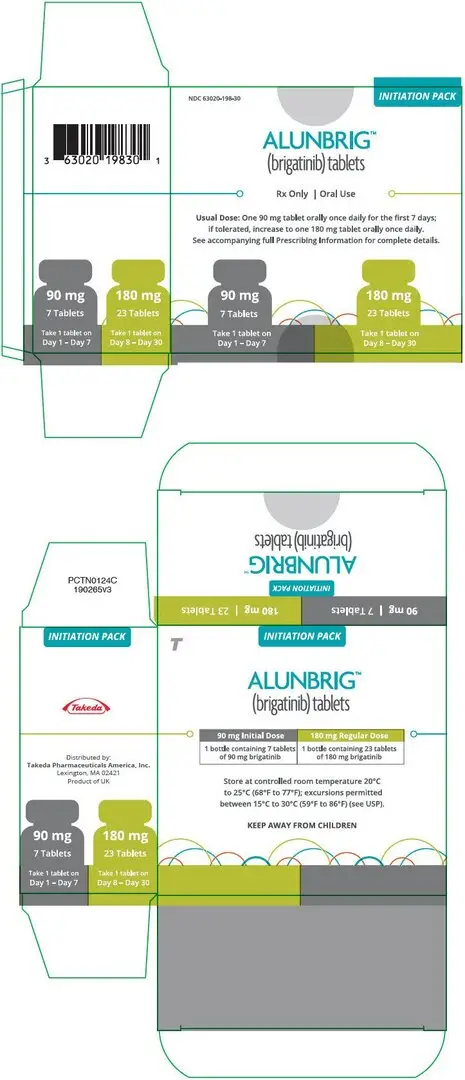
90 mg / 7 count tablets (NDC 63020-090-07) and 180 mg / 23 count tablets (NDC 63020-180-23) are also available in a single carton as a one-month initiation pack:
| One carton containing one bottle of 90 mg tablets (7 count) and one bottle of 180 mg tablets (23 count) | NDC 63020-198-30 |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| PATIENT INFORMATION ALUNBRIG (uh-lun-brig) (brigatinib) tablets |
|||||
|---|---|---|---|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised: February 2022 | ||||
| What is the most important information I should know about ALUNBRIG? ALUNBRIG can cause serious side effects, including:
|
|||||
|
|
||||
|
|||||
|
|
||||
|
|||||
|
|
||||
|
|||||
|
|
||||
| See "What are the possible side effects of ALUNBRIG?" for information about side effects. | |||||
| What is ALUNBRIG?
ALUNBRIG is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC):
|
|||||
Before you take ALUNBRIG, tell your healthcare provider about all of your medical conditions, including if you:
|
|||||
How should I take ALUNBRIG?
|
|||||
What should I avoid while taking ALUNBRIG?
|
|||||
| What are the possible side effects of ALUNBRIG? ALUNBRIG may cause serious side effects, including:
|
|||||
|
|
|
|||
| ALUNBRIG may cause fertility problems in males. This may affect your ability to father a child. Talk to your healthcare provider if you have concerns about fertility. These are not all of the possible side effects of ALUNBRIG. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||
How should I store ALUNBRIG?
|
|||||
| General information about the safe and effective use of ALUNBRIG.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ALUNBRIG for a condition for which it was not prescribed. Do not give ALUNBRIG to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ALUNBRIG that is written for health professionals. |
|||||
| What are the ingredients in ALUNBRIG? Active ingredient: brigatinib Inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate (Type A), magnesium stearate, and hydrophobic colloidal silica. The tablet coating consists of talc, polyethylene glycol, polyvinyl alcohol, and titanium dioxide. Distributed by: Takeda Pharmaceuticals America, Inc., Lexington, MA 02421 ALUNBRIG® is a registered trademark of ARIAD Pharmaceuticals, Inc. ©2022 ARIAD Pharmaceuticals, Inc. All rights reserved. For more information, go to www.alunbrig.com or call 1-844-217-6468. ABG346 R5 |
|||||
| ALUNBRIG
brigatinib tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ALUNBRIG
brigatinib tablet, film coated |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ALUNBRIG
brigatinib tablet, film coated |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ALUNBRIG
brigatinib kit |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMPAC Fine Chemicals LLC | 073903937 | API MANUFACTURE(63020-113, 63020-090, 63020-180, 63020-198) , ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia New York, Inc. | 124193793 | ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) , API MANUFACTURE(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Curia Indiana, LLC | 020593403 | ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ajinomoto Omnichem | 400344443 | API MANUFACTURE(63020-113, 63020-090, 63020-180, 63020-198) , ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Solvias AG | 480739627 | ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Penn Pharmaceutical Services Limited | 226277259 | MANUFACTURE(63020-113, 63020-090, 63020-180, 63020-198) , ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) , PACK(63020-113, 63020-090, 63020-180, 63020-198) , LABEL(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Almac Sciences (Ireland) Limited | 985822621 | ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda Ireland Limited | 988980314 | MANUFACTURE(63020-113, 63020-090, 63020-180, 63020-198) , ANALYSIS(63020-113, 63020-090, 63020-180, 63020-198) , PACK(63020-113, 63020-090, 63020-180, 63020-198) , LABEL(63020-113, 63020-090, 63020-180, 63020-198) | |