Drug Detail:Amitiza (Lubiprostone [ loo-bee-pros-tone ])
Drug Class: Chloride channel activators
Highlights of Prescribing Information
AMITIZA (lubiprostone) capsules, for oral use
Initial U.S. Approval: 2006
Indications and Usage for Amitiza
Amitiza is a chloride channel activator indicated for the treatment of:
- chronic idiopathic constipation (CIC) in adults. (1.1)
- opioid-induced constipation (OIC) in adult patients with chronic, non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation. (1.2)
-
Limitations of Use:
Effectiveness of Amitiza in the treatment of OIC in patients taking diphenylheptane opioids (e.g., methadone) has not been established. (1.2, 7.1)
-
Limitations of Use:
- irritable bowel syndrome with constipation (IBS-C) in women ≥ 18 years old. (1.3)
Amitiza Dosage and Administration
Recommended Dosage (2.1)
- CIC and OIC: 24 mcg twice daily.
- IBS-C: 8 mcg twice daily.
- See full prescribing information for dosage adjustment by indication and degree of hepatic impairment.
Administration Instructions (2.2)
- Swallow capsules whole and do not break apart or chew,
- Take capsules with food and water,
- Assess periodically the need for continuous therapy.
Dosage Forms and Strengths
Capsules: 8 mcg and 24 mcg (3)
Contraindications
Patients with known or suspected mechanical gastrointestinal obstruction. (4, 5.5)
Warnings and Precautions
- Nausea: Patients may experience nausea; concomitant administration of food may reduce this symptom. (2.2, 5.1)
- Diarrhea: Avoid use in patients with severe diarrhea. Instruct patients to discontinue Amitiza and contact their healthcare provider if severe diarrhea occurs during treatment. (5.2)
- Syncope and Hypotension: May occur after taking the first dose or with subsequent doses. Generally resolves prior to the next dose, but may recur with repeat dosing. Instruct patients to discontinue Amitiza and contact their healthcare provider if symptoms occur. (5.3)
- Dyspnea: May occur within an hour of first dose. Generally resolves within 3 hours, but may recur with repeat dosing. Instruct patients to contact their healthcare provider if symptoms occur. (5.4)
- Bowel Obstruction: Evaluate patients with symptoms suggestive of mechanical gastrointestinal obstruction prior to initiating treatment with Amitiza. (4, 5.5)
Adverse Reactions/Side Effects
Most common adverse reactions (> 4%) are:
- CIC: nausea, diarrhea, headache, abdominal pain, abdominal distension, and flatulence. (6.1)
- OIC: nausea and diarrhea. (6.1)
- IBS-C: nausea, diarrhea, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-877-825-3327 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Pediatrics: Safety and effectiveness have not been established in pediatric patients with IBS-C, pediatric functional constipation (PFC), and OIC. (8.4)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2020
Full Prescribing Information
1. Indications and Usage for Amitiza
1.1 Chronic Idiopathic Constipation in Adults
Amitiza® is indicated for the treatment of chronic idiopathic constipation (CIC) in adults.
1.2 Opioid-Induced Constipation in Adult Patients with Chronic Non-Cancer Pain
Amitiza is indicated for the treatment of opioid-induced constipation (OIC) in adult patients with chronic non-cancer pain, including patients with chronic pain related to prior cancer or its treatment who do not require frequent (e.g., weekly) opioid dosage escalation.
2. Amitiza Dosage and Administration
2.1 Recommended Dosage
The recommended oral dosage of Amitiza by indication and adjustments for patients with moderate (Child Pugh Class B) and severe (Child Pugh Class C) hepatic impairment are shown in Table 1.
| CIC and OIC | IBS-C | |
|---|---|---|
|
||
| Recommended Adult Dosage Regimen | 24 mcg twice daily | 8 mcg twice daily |
| Dosage Adjustment for Hepatic Impairment [see Use in Specific Populations (8.6)] | Moderate Impairment (Child-Pugh Class B): 16 mcg twice daily* | Moderate Impairment (Child-Pugh Class B): No adjustment necessary |
| Severe Impairment (Child-Pugh Class C): 8 mcg twice daily* | Severe Impairment (Child-Pugh Class C): 8 mcg once daily* |
|
3. Dosage Forms and Strengths
Amitiza is available as an oval, gelatin capsule containing 8 mcg or 24 mcg of lubiprostone.
- 8 mcg capsules are pink and are printed with "SPI" on one side
- 24 mcg capsules are orange and are printed with "SPI" on one side
4. Contraindications
Amitiza is contraindicated in patients with known or suspected mechanical gastrointestinal obstruction [see Warnings and Precautions (5.5)].
5. Warnings and Precautions
5.1 Nausea
Patients taking Amitiza may experience nausea. Concomitant administration of food with Amitiza may reduce symptoms of nausea [see Adverse Reactions (6.1)].
5.2 Diarrhea
Avoid use of Amitiza in patients with severe diarrhea. Patients should be aware of the possible occurrence of diarrhea during treatment. Instruct patients to discontinue Amitiza and contact their healthcare provider if severe diarrhea occurs [see Adverse Reactions (6.1)].
5.3 Syncope and Hypotension
Syncope and hypotension have been reported with Amitiza in the postmarketing setting and a few of these adverse reactions resulted in hospitalization. Most cases occurred in patients taking 24 mcg twice daily and some occurred within an hour after taking the first dose or subsequent doses of Amitiza. Some patients had concomitant diarrhea or vomiting prior to developing the adverse reaction. Syncope and hypotension generally resolved following Amitiza discontinuation or prior to next dose, but recurrence has been reported with subsequent doses. Several cases reported concomitant use of medications known to lower blood pressure, which may increase the risk for the development of syncope or hypotension.
Patients should be aware of the risk of syncope and hypotension during treatment and that other adverse reactions may increase this risk, such as diarrhea or vomiting.
5.4 Dyspnea
In clinical trials, dyspnea was reported by 3%, 1%, and < 1% of the treated CIC, OIC, and IBS-C populations receiving Amitiza, respectively, compared to 0%, 1%, and < 1% of placebo-treated patients. There have been postmarketing reports of dyspnea when using Amitiza 24 mcg twice daily. Some patients have discontinued treatment because of dyspnea. These events have usually been described as a sensation of chest tightness and difficulty taking in a breath, and generally have an acute onset within 30 to 60 minutes after taking the first dose. They generally resolve within a few hours after taking the dose, but recurrence has been frequently reported with subsequent doses. Instruct patients to contact their healthcare provider if dyspnea occurs.
6. Adverse Reactions/Side Effects
The following adverse reactions are described below and elsewhere in labeling:
- Nausea [see Warnings and Precautions (5.1)]
- Diarrhea [see Warnings and Precautions (5.2)]
- Syncope and Hypotension [see Warnings and Precautions (5.3)]
- Dyspnea [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
During clinical development of Amitiza for CIC, OIC, and IBS-C, 1648 patients were treated with Amitiza for 6 months and 710 patients were treated for 1 year (not mutually exclusive).
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of Amitiza. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular: syncope and/or hypotension [see Warnings and Precautions (5.3)], tachycardia
Gastrointestinal: ischemic colitis
General: asthenia
Immune System: hypersensitivity reactions including rash, swelling, and throat tightness malaise
Muscoskeletal: muscle cramps or muscle spasms.
7. Drug Interactions
7.1 Methadone
Diphenylheptane opioids (e.g., methadone) have been shown in nonclinical studies to dose-dependently reduce the activation of ClC-2 by lubiprostone in the gastrointestinal tract. There is a possibility of a dose-dependent decrease in the efficacy of Amitiza in patients using diphenylheptane opioids. No in vivo interaction studies have been conducted.
The effectiveness of Amitiza in the treatment of OIC in patients taking diphenylhepatane opioids (e.g., methadone) has not been established [see Indications and Usage (1.2)].
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients with IBS-C, pediatric functional constipation (PFC), and OIC.
Efficacy was not demonstrated for the treatment of PFC in patients 6 years of age and older in a 12 week, randomized, double-blind, placebo-controlled trial conducted in 606 patients 6 to 17 years with PFC comparing Amitiza to placebo. The primary efficacy endpoint was an overall response based on spontaneous bowel movement frequency over the duration of the trial; the treatment difference from placebo was not statistically significant. In this age group, adverse reactions to Amitiza were similar to those reported in adults. In a 36-week, long-term safety extension trial after approximately 9 months of treatment with Amitiza, a single case of reversible elevation of ALT (17-times upper limit of normal [ULN]), AST (13-times ULN), and GGT (9-times [ULN]) was observed in a child with baseline elevated values (less than or equal to 2.5-times ULN).
8.6 Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh Class B) and severe hepatic impairment (Child-Pugh Class C) experienced markedly higher systemic exposure of lubiprostone active metabolite M3, when compared to subjects with normal hepatic function [see Clinical Pharmacology (12.3)]. Clinical safety results demonstrated an increased incidence and severity of adverse events in subjects with greater severity of hepatic impairment.
Adjust the dosage of Amitiza in patients with severe hepatic impairment for all indications. Dosage adjustment is also needed for patients with moderate hepatic impairment treated for CIC, and OIC [see Dosage and Administration (2.1)]. No dosing adjustment is required in patients with mild hepatic impairment (Child-Pugh Class A).
10. Overdosage
There have been six reports of overdosage with Amitiza during clinical development. Of these six cases, only two subjects reported adverse events: one reported vomiting, diarrhea and stomach ache after taking 168 to 192 mcg of Amitiza, and another reported diarrhea and a joint injury on the day of overdose after taking 36 mcg of Amitiza. Adverse reactions that occurred in at least 1% of healthy subjects given a single oral dose of 144 mcg of Amitiza (6 times the highest recommended dose) in a cardiac repolarization study included nausea (45%), diarrhea (35%), vomiting (27%), dizziness (14%), headache (12%), abdominal pain (8%), flushing/hot flash (8%), retching (8%), dyspnea (4%), pallor (4%), stomach discomfort (4%), anorexia (2%), asthenia (2%), chest discomfort (2%), dry mouth (2%), hyperhidrosis (2%), and syncope (2%).
11. Amitiza Description
Amitiza (lubiprostone) is a chloride channel activator for oral use.
The chemical name for lubiprostone is (–)-7-[(2R,4aR,5R,7aR)-2-(1,1-difluoropentyl)-2-hydroxy-6-oxooctahydrocyclopenta[b]pyran-5-yl]heptanoic acid. The molecular formula of lubiprostone is C20H32F2O5 with a molecular weight of 390.46 and a chemical structure as follows:
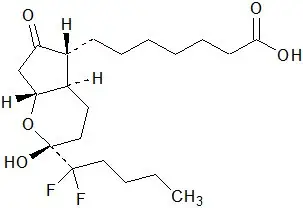
Lubiprostone drug substance occurs as white, odorless crystals or crystalline powder, is very soluble in ether and ethanol, and is practically insoluble in hexane and water. Amitiza is available as an imprinted, oval, soft gelatin capsule in two strengths. Pink capsules contain 8 mcg of lubiprostone and the following inactive ingredients: ferric oxide, gelatin, medium-chain triglycerides, purified water, sorbitol, and titanium dioxide. Orange capsules contain 24 mcg of lubiprostone and the following inactive ingredients: D&C Yellow #10, FD&C Red #40, gelatin, medium-chain triglycerides, purified water, and sorbitol.
12. Amitiza - Clinical Pharmacology
12.1 Mechanism of Action
Lubiprostone is a locally acting chloride channel activator that enhances a chloride-rich intestinal fluid secretion without altering sodium and potassium concentrations in the serum. Lubiprostone acts by specifically activating ClC-2, which is a normal constituent of the apical membrane of the human intestine, in a protein kinase A–independent fashion.
By increasing intestinal fluid secretion, lubiprostone increases motility in the intestine, thereby facilitating the passage of stool and alleviating symptoms associated with chronic idiopathic constipation. Patch clamp cell studies in human cell lines have indicated that the majority of the beneficial biological activity of lubiprostone and its metabolites is observed only on the apical (luminal) portion of the gastrointestinal epithelium.
Lubiprostone, via activation of apical ClC-2 channels in intestinal epithelial cells, bypasses the antisecretory action of opiates that results from suppression of secretomotor neuron excitability.
Activation of ClC-2 by lubiprostone has also been shown to stimulate recovery of mucosal barrier function and reduce intestinal permeability via the restoration of tight junction protein complexes in ex vivo studies of ischemic porcine intestine.
12.2 Pharmacodynamics
Although the pharmacologic effects of lubiprostone in humans have not been fully evaluated, animal studies have shown that oral administration of lubiprostone increases chloride ion transport into the intestinal lumen, enhances fluid secretion into the bowels, and improves fecal transit.
12.3 Pharmacokinetics
Following oral administration, concentrations of lubiprostone in plasma are below the level of quantitation (10 pg/mL). Therefore, standard pharmacokinetic parameters such as area under the curve (AUC), maximum concentration (Cmax), and half-life (t½) cannot be reliably calculated. However, the pharmacokinetic parameters of M3 (only measurable active metabolite of lubiprostone) have been characterized.
14. Clinical Studies
14.1 Chronic Idiopathic Constipation in Adults
Two double-blinded, placebo-controlled studies of identical design were conducted in patients with CIC. CIC was defined as, on average, less than 3 SBMs per week (a SBM is a bowel movement occurring in the absence of laxative use) along with one or more of the following symptoms of constipation for at least 6 months prior to randomization: 1) very hard stools for at least a quarter of all bowel movements; 2) sensation of incomplete evacuation following at least a quarter of all bowel movements; and 3) straining with defecation at least a quarter of the time.
Following a 2-week baseline/washout period, a total of 479 patients (mean age 47 [range 20 to 81] years; 89% female; 81% Caucasian, 10% African American, 7% Hispanic, 2% Asian, 11% at least 65 years of age) were randomized and received Amitiza 24 mcg twice daily or placebo twice daily for 4 weeks. The primary endpoint of the studies was SBM frequency. The studies demonstrated that patients treated with Amitiza had a higher frequency of SBMs during Week 1 than the placebo patients. In both studies, results similar to those in Week 1 were also observed in Weeks 2, 3, and 4 of therapy (Table 6).
| Trial | Study Arm | Baseline Mean ± SD Median | Week 1 Mean ± SD Median | Week 2 Mean ± SD Median | Week 3 Mean ± SD Median | Week 4 Mean ± SD Median | Week 1 Change from Baseline Mean ± SD Median | Week 4 Change from Baseline Mean ± SD Median |
|---|---|---|---|---|---|---|---|---|
|
||||||||
| Study 1 | Placebo | 1.6 ± 1.3 1.5 | 3.5 ± 2.3 3.0 | 3.2 ± 2.5 3.0 | 2.8 ± 2.2 2.0 | 2.9 ± 2.4 2.3 | 1.9 ± 2.2 1.5 | 1.3 ± 2.5 1.0 |
| Amitiza 24 mcg Twice Daily | 1.4 ± 0.8 1.5 | 5.7 ± 4.4 5.0 | 5.1 ± 4.1 4.0 | 5.3 ± 4.9 5.0 | 5.3 ± 4.7 4.0 | 4.3 ± 4.3 3.5 | 3.9 ± 4.6 3.0 |
|
| Study 2 | Placebo | 1.5 ± 0.8 1.5 | 4.0 ± 2.7 3.5 | 3.6 ± 2.7 3.0 | 3.4 ± 2.8 3.0 | 3.5 ± 2.9 3.0 | 2.5 ± 2.6 1.5 | 1.9 ± 2.7 1.5 |
| Amitiza 24 mcg Twice Daily | 1.3 ± 0.9 1.5 | 5.9 ± 4.0 5.0 | 5.0 ± 4.2 4.0 | 5.6 ± 4.6 5.0 | 5.4 ± 4.8 4.3 | 4.6 ± 4.1 3.8 | 4.1 ± 4.8 3.0 |
|
In both studies, Amitiza demonstrated increases in the percentage of patients who experienced SBMs within the first 24 hours after administration when compared to placebo (57% vs. 37% in Study 1 and 63% vs. 32% in Study 2, respectively). Similarly, the time to first SBM was shorter for patients receiving Amitiza than for those receiving placebo.
Signs and symptoms related to constipation, including abdominal bloating, abdominal discomfort, stool consistency, and straining, as well as constipation severity ratings, were also improved with Amitiza versus placebo. The results were consistent in subpopulation analyses for gender, race, and elderly patients at least 65 years of age.
During a 7-week randomized withdrawal study, patients who received Amitiza during a 4-week treatment period were then randomized to receive either placebo or to continue treatment with Amitiza. In Amitiza-treated patients randomized to placebo, SBM frequency rates returned toward baseline within 1 week and did not result in worsening compared to baseline. Patients who continued on Amitiza maintained their response to therapy over the additional 3 weeks of treatment.
14.2 Opioid-Induced Constipation in Adults with Chronic Non-Cancer Pain
The efficacy of Amitiza in the treatment of OIC in patients receiving opioid therapy for chronic, non-cancer-related pain was assessed in three randomized, double-blinded, placebo-controlled studies. In Study 1, the median age was 52 years (range 20 to 82) and 63% were female. In Study 2, the median age was 50 years (range 21 to 77) and 64% were female. In Study 3, the median age was 50 years (range 21 to 89) and 60% were female. Patients had been receiving stable opioid therapy for at least 30 days prior to screening, which was to continue throughout the 12-week treatment period. At baseline, mean oral morphine equivalent daily doses (MEDDs) were 99 mg and 130 mg for placebo-treated and Amitiza-treated patients, respectively, in Study 1. Baseline mean MEDDs were 237 mg and 265 mg for placebo-treated and Amitiza-treated patients, respectively, in Study 2. In Study 3, baseline mean MEDDs were 330 mg and 373 mg for placebo-treated and Amitiza-treated patients, respectively. The Brief Pain Inventory-Short Form (BPI-SF) questionnaire was administered to patients at baseline and monthly during the treatment period to assess pain control. Patients had documented opioid-induced constipation at baseline, defined as having less than 3 spontaneous bowel movements (SBMs) per week, with at least 25% of SBMs associated with one or more of the following conditions: (1) hard to very hard stool consistency; (2) moderate to very severe straining; and/or (3) having a sensation of incomplete evacuation. Laxative use was discontinued at the beginning of the screening period and throughout the study. With the exception of the 48-hour period prior to first dose and for at least 72 hours (Study 1) or 1 week (Study 2 and Study 3) following first dose, use of rescue medication was allowed in cases where no bowel movement had occurred in a 3-day period. Median weekly SBM frequencies at baseline were 1.5 for placebo patients and 1.0 for Amitiza patients in Study 1 and, for both Study 2 and Study 3, median weekly SBM frequencies at baseline were 1.5 for both treatment groups.
In Study 1, patients receiving non-diphenylheptane (e.g., non-methadone) opioids (n = 431) were randomized to receive placebo (n = 217) or Amitiza 24 mcg twice daily (n = 214) for 12 weeks. The primary efficacy analysis was a comparison of the proportion of "overall responders" in each treatment arm. A patient was considered an "overall responder" if ≥1 SBM improvement over baseline were reported for all treatment weeks for which data were available and ≥3 SBMs/week were reported for at least 9 of 12 treatment weeks. The proportion of patients in Study 1 qualifying as an "overall responder" was 27.1% in the group receiving Amitiza 24 mcg twice daily compared to 18.9% of patients receiving placebo twice daily (treatment difference = 8.2%; p-value = 0.03). Examination of gender and race subgroups did not identify differences in response to Amitiza among these subgroups. There were too few elderly patients (≥ 65 years of age) to adequately assess differences in effects in that population.
In Study 2, patients receiving opioids (N = 418) were randomized to receive placebo (n = 208) or Amitiza 24 mcg twice daily (n = 210) for 12 weeks. Study 2 did not exclude patients receiving diphenylheptane opioids (e.g., methadone). The primary efficacy endpoint was the mean change from baseline in SBM frequency at Week 8; 3.3 vs. 2.4 for Amitiza and placebo-treated patients, respectively; treatment difference = 0.9; p-value = 0.004. The proportion of patients in Study 2 qualifying as an "overall responder," as prespecified in Study 1, was 24% in the group receiving Amitiza compared to 15% of patients receiving placebo. In the subgroup of patients in Study 2 taking diphenylheptane opioids (baseline mean [median] MEDDs of 691 [403] mg and 672 [450] mg for placebo and Amitiza patients, respectively), the proportion of patients qualifying as an "overall responder" was 20.5% (8/39) in the group receiving Amitiza compared to 6.3% (2/32) of patients receiving placebo. Examination of gender and race subgroups did not identify differences in response to Amitiza among these subgroups. There were too few elderly patients (≥ 65 years of age) to adequately assess differences in effects in that population.
In Study 3, patients receiving opioids (N = 451) were randomized to placebo (n = 216) or Amitiza 24 mcg twice daily (n = 235) for 12 weeks. Study 3 did not exclude patients receiving diphenylheptane opioids (e.g., methadone). The primary efficacy endpoint was the change from baseline in SBM frequency at Week 8. The study did not demonstrate a statistically significant improvement in SBM frequency rates at Week 8 (mean change from baseline of 2.7 vs. 2.5 for Amitiza and placebo-treated patients, respectively; treatment difference = 0.2; p-value = 0.76). The proportion of patients in Study 3 qualifying as an "overall responder," as prespecified in Study 1, was 15% in the patients receiving Amitiza compared to 13% of patients receiving placebo. In the subgroup of patients in Study 3 taking diphenylheptane opioids (baseline mean [median] MEDDs of 730 [518] mg and 992 [480] mg for placebo and Amitiza patients, respectively), the proportion of patients qualifying as an "overall responder" was 2% (1/47) in the group receiving Amitiza compared to 12% (5/41) of patients receiving placebo.
14.3 Irritable Bowel Syndrome with Constipation
Two double-blinded, placebo-controlled studies of similar design were conducted in adult patients with IBS-C. IBS was defined as abdominal pain or discomfort occurring over at least 6 months with two or more of the following: 1) relieved with defecation; 2) onset associated with a change in stool frequency; and 3) onset associated with a change in stool form. Patients were sub-typed as having IBS-C if they also experienced two of three of the following: 1) <3 spontaneous bowel movements (SBMs) per week, 2) >25% hard stools, and 3) >25% SBMs associated with straining.
Following a 4-week baseline/washout period, a total of 1154 patients (mean age 47 [range 18 to 85] years; 92% female; 77% Caucasian, 13% African American, 9% Hispanic, 0.4% Asian; 8% at least 65 years of age) were randomized and received Amitiza 8 mcg twice daily (16 mcg/day) or placebo twice daily for 12 weeks. The primary efficacy endpoint was assessed weekly utilizing the patient's response to a global symptom relief question based on a 7-point, balanced scale ("significantly worse" to "significantly relieved"): "How would you rate your relief of IBS symptoms (abdominal discomfort/pain, bowel habits, and other IBS symptoms) over the past week compared to how you felt before you entered the study?"
The primary efficacy analysis was a comparison of the proportion of "overall responders" in each arm. A patient was considered an "overall responder" if the criteria for being designated a "monthly responder" were met in at least 2 of the 3 months on study. A "monthly responder" was defined as a patient who had reported "significantly relieved" for at least 2 weeks of the month or at least "moderately relieved" in all 4 weeks of that month. During each monthly evaluation period, patients reporting "moderately worse" or "significantly worse" relief, an increase in rescue medication use, or those who discontinued due to lack of efficacy, were deemed non-responders.
The percentage of patients in Study 1 qualifying as an "overall responder" was 14% in the group receiving Amitiza 8 mcg twice daily compared to 8% of patients receiving placebo twice daily. In Study 2, 12% of patients in the Amitiza 8 mcg group were "overall responders" versus 6% of patients in the placebo group. In both studies, the treatment differences between the placebo and Amitiza groups were statistically significant.
16. How is Amitiza supplied
Amitiza is available as an oval, soft gelatin capsule containing 8 mcg or 24 mcg of lubiprostone with "SPI" printed on one side. Amitiza is available as follows:
8 mcg pink capsule
- Bottles of 60 (NDC 64764-080-60)
24 mcg orange capsule
- Bottles of 60 (NDC 64764-240-60)
17. Patient Counseling Information
Administration Instructions
- Instruct patients to take Amitiza orally with food and water to reduce the occurrence of nausea [see Warnings and Precautions (5.1)].
- Swallow capsules whole and do not break apart or chew.
- Physicians and patients should periodically assess the need for continued therapy.
| AMITIZA
lubiprostone capsule, gelatin coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| AMITIZA
lubiprostone capsule, gelatin coated |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |
| Registrant - Sucampo Pharma Americas, LLC (117587624) |






