Drug Detail:Aptensio xr (Methylphenidate (oral) [ meth-il-fen-i-date ])
Drug Class: CNS stimulants
Highlights of Prescribing Information
APTENSIO XR® (methylphenidate hydrochloride extended-release) capsules, for oral use, CII
Initial U.S. Approval: 1955
WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning.
- CNS stimulants, including APTENSIO XR, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence. (5.1, 9.2, 9.3)
- Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy. (5.1, 9.2, 9.3)
Indications and Usage for Aptensio XR
APTENSIO XR is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older. (1)
Limitations of Use:
Pediatric patients younger than 6 years of age experienced higher plasma exposure than patients 6 years and older at the same dose and high rates of adverse reactions, most notably weight loss. (8.4)
Aptensio XR Dosage and Administration
- Recommended starting dose for patients 6 years and older: 10 mg once daily with or without food in the morning. Dosage may be increased weekly in increments of 10 mg per day. Daily dosage above 60 mg is not recommended. (2.1)
- Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce. (2.1)
Dosage Forms and Strengths
Extended-Release Capsules: 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 50 mg, and 60 mg of methylphenidate hydrochloride, which is equivalent to 8.6 mg, 13.0 mg, 17.3 mg, 25.9 mg, 34.6 mg, 43.2 mg, and 51.9 mg of methylphenidate free base, respectively, per capsule. (3)
Contraindications
- Known hypersensitivity to methylphenidate or product components. (4)
- Concurrent treatment with a monoamine oxidase inhibitor (MAOI), or use of an MAOI within the preceding 14 days. (4)
Warnings and Precautions
- Serious Cardiovascular Events: Sudden death has been reported in association with CNS stimulant treatment at recommended doses in pediatric patients with structural cardiac abnormalities or other serious heart problems. In adults, sudden death, stroke, and myocardial infarction have been reported. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmias, or coronary artery disease. (5.2)
- Blood Pressure and Heart Rate Increases: Monitor blood pressure and pulse. Consider the benefits and risks in patients for whom an increase in blood pressure or heart rate would be problematic. (5.3)
- Psychiatric Adverse Reactions: Use of stimulants may cause psychotic or manic symptoms in patients with no prior history, or exacerbation of symptoms in patients with pre-existing psychiatric illness. Evaluate for bipolar disorder prior to APTENSIO XR use. (5.4)
- Priapism: Cases of painful and prolonged penile erections and priapism have been reported with methylphenidate products. Immediate medical attention should be sought if signs or symptoms of prolonged penile erections or priapism are observed. (5.5)
- Peripheral Vasculopathy, including Raynaud's Phenomenon: Stimulants used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Careful observation for digital changes is necessary during treatment with ADHD stimulants. (5.6)
- Long-Term Suppression of Growth: Monitor height and weight at appropriate intervals in pediatric patients. (5.7)
Adverse Reactions/Side Effects
The most common adverse reactions in double-blind clinical trials (> 5% and twice the rate of placebo) in pediatric patients 6 to 17 years were abdominal pain, decreased appetite, headache and insomnia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rhodes Pharmaceuticals at (1-888-827-0616); or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Drug Interactions
Antihypertensive drugs: Monitor blood pressure. Adjust dosage of antihypertensive drug as needed (7).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2023
Related/similar drugs
Adderall, Vyvanse, methylphenidate, Strattera, Ritalin, ConcertaFull Prescribing Information
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including APTENSIO XR, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy [see Warning and Precautions (5.1), Drug Abuse and Dependence (9.2, 9.3)].
1. Indications and Usage for Aptensio XR
APTENSIO XR is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older [see Clinical Studies (14)].
2. Aptensio XR Dosage and Administration
2.1 Pretreatment Screening
Prior to treating pediatric patients and adults with CNS stimulants including APTENSIO XR, assess for the presence of cardiac disease (i.e., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions 5.2].
Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy. Maintain careful prescription records, educate patients about abuse, monitor for signs of abuse and overdose, and periodically re-evaluate the need for APTENSIO XR use [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9)].
2.2 General Dosing Information
The recommended starting dose of APTENSIO XR for patients 6 years and older is 10 mg once daily in the morning with or without food. Advise patients to establish a routine pattern with regard to meals. The dose should be individualized according to the needs and response of the patient.
The dose may be titrated weekly in increments of 10 mg. Daily doses above 60 mg have not been studied and are not recommended.
APTENSIO XR may be taken whole or the capsule may be opened and the entire contents sprinkled onto applesauce. If the patient is using the sprinkled administration method, the sprinkled applesauce should be consumed immediately; it should not be stored. Patients should take the applesauce with sprinkled beads in its entirety without chewing. The dose of a single capsule should not be divided. The contents of the entire capsule should be taken, and patients should not take anything less than one capsule per day.
Pharmacological treatment of ADHD may be needed for extended periods. Healthcare providers should periodically re-evaluate the long-term use of APTENSIO XR, and adjust dosage as needed.
2.3 Dose Reduction and Discontinuation
If paradoxical aggravation of symptoms or other adverse reactions occur; the dosage should be reduced, or, if necessary, the drug should be discontinued.
If improvement is not observed after appropriate dosage adjustment over a one-month period, the drug should be discontinued.
3. Dosage Forms and Strengths
-
10 mg Extended-Release Capsules – light turquoise blue cap/white body
(imprinted with "APTENSIO XR" on cap and "10 mg" on the body) -
15 mg Extended-Release Capsules – orange cap/white body
(imprinted with "APTENSIO XR" on cap and "15 mg" on the body) -
20 mg Extended-Release Capsules – yellow cap/white body
(imprinted with "APTENSIO XR" on cap and "20 mg" on the body) -
30 mg Extended-Release Capsules – blue violet cap/white body
(imprinted with "APTENSIO XR" on cap and "30 mg" on the body) -
40 mg Extended-Release Capsules – pink cap/white body
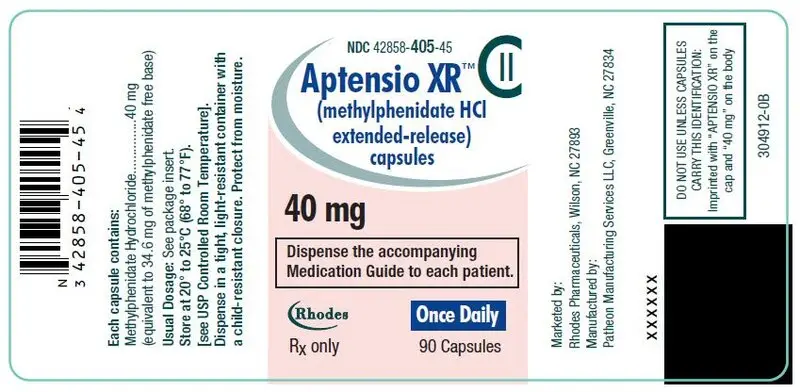
(imprinted with "APTENSIO XR" on cap and "40 mg" on the body) -
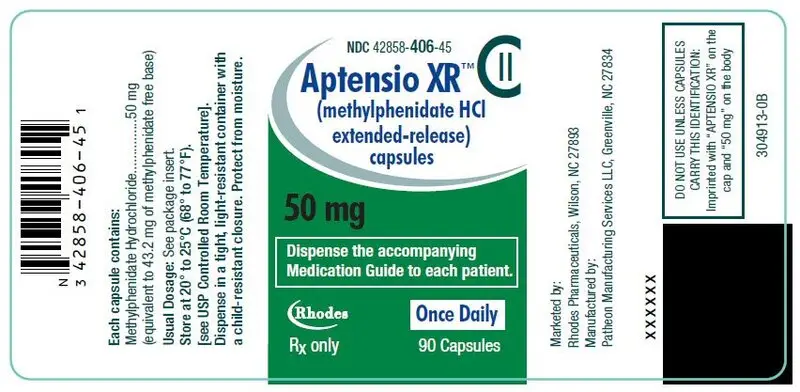
50 mg Extended-Release Capsules – green cap/white body
(imprinted with "APTENSIO XR" on cap and "50 mg" on the body) -
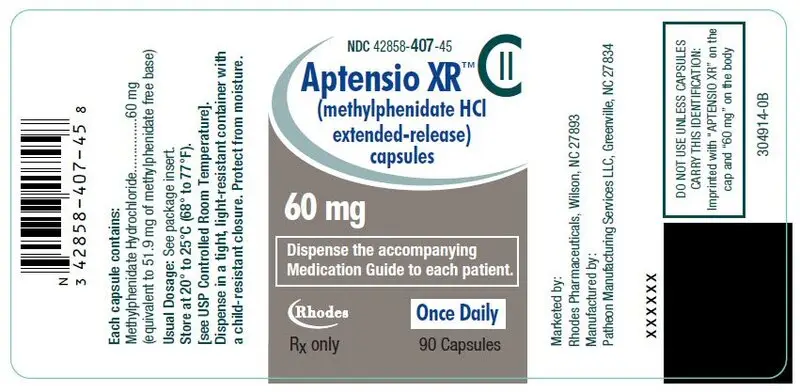
60 mg Extended-Release Capsules – gray cap/white body
(imprinted with "APTENSIO XR" on cap and "60 mg" on the body)
4. Contraindications
- Hypersensitivity to methylphenidate or other components of the product. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with methylphenidate products [see Adverse Reactions (6.1)].
- Concomitant treatment with monoamine oxidase inhibitors, and also within 14 days following discontinuation of treatment with a monoamine oxidase inhibitor, because of the risk of hypertensive crisis [see Drug Interactions (7.1)].
5. Warnings and Precautions
5.1 Potential for Abuse and Dependence
CNS stimulants, including APTENSIO XR, other methylphenidate-containing products, and amphetamines, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy [see Boxed Warning and Drug Abuse and Dependence (9.2, 9.3)].
5.2 Serious Cardiovascular Reactions
Sudden death, stroke and myocardial infarction have been reported in adults with CNS stimulant treatment at recommended doses. Sudden death has been reported in pediatric patients with structural cardiac abnormalities and other serious heart problems taking CNS stimulants at recommended doses for ADHD. Avoid use in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart arrhythmia, coronary artery disease, and other serious heart problems. Further evaluate patients who develop exertional chest pain, unexplained syncope, or arrhythmias during APTENSIO XR treatment.
5.3 Blood Pressure and Heart Rate Increases
CNS stimulants cause an increase in blood pressure (mean increase approximately 2 to 4 mmHg) and heart rate (mean increase approximately 3 to 6 bpm). Individuals may have larger increases. Monitor all patients for hypertension and tachycardia.
5.5 Priapism
Prolonged and painful erections, sometimes requiring surgical intervention, have been reported with methylphenidate products, in both pediatric and adult patients. Priapism was not reported with drug initiation but developed after some time on the drug, often subsequent to an increase in dose. Priapism has also appeared during a period of drug withdrawal (drug holidays or during discontinuation). Patients who develop abnormally sustained or frequent and painful erections should seek immediate medical attention.
5.6 Peripheral Vasculopathy, including Raynaud's Phenomenon
CNS stimulants, including APTENSIO XR, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.7 Long-Term Suppression of Growth
CNS stimulants have been associated with weight loss and slowing of growth rate in pediatric patients.
Careful follow-up of weight and height in pediatric patients ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated pediatric patients over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated pediatric patients (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development.
Closely monitor growth (weight and height) in pediatric patients treated with CNS stimulants, including APTENSIO XR. Patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
6. Adverse Reactions/Side Effects
The following are discussed in more detail in other sections of the labeling:
- Abuse and Dependence [see Boxed Warning, Warnings and Precautions (5.1), and Drug Abuse and Dependence (9.2, 9.3)]
- Hypersensitivity to Methylphenidate [see Contraindications (4)]
- Hypertensive Crisis with Concomitant Use of Monoamine Oxidase Inhibitors [see Contraindications (4) and Drug Interactions (7.1)]
- Serious Cardiovascular Reactions [see Warnings and Precautions (5.2)]
- Blood Pressure and Heart Rate Increases [see Warnings and Precautions (5.3)]
- Psychiatric Adverse Reactions [see Warnings and Precautions (5.4)]
- Priapism [see Warnings and Precautions (5.5)]
- Peripheral Vasculopathy, including Raynaud's Phenomenon [see Warnings and Precautions (5.6)]
- Long-Term Suppression of Growth [see Warnings and Precautions (5.7)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of methylphenidate products. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These adverse reactions are as follows:
Blood and Lymphatic System Disorders: Pancytopenia, Thrombocytopenia, Thrombocytopenic purpura
Cardiac Disorders: Angina pectoris, Bradycardia, Extrasystole, Supraventricular tachycardia, Ventricular extrasystole
Eye Disorders: Diplopia, Mydriasis, Visual impairment
General Disorders: Chest pain, Chest discomfort, Hyperpyrexia
Immune System Disorders: Hypersensitivity reactions such as Angioedema, Anaphylactic reactions, Auricular swelling, Bullous conditions, Exfoliative conditions, Urticarias, Pruritus NEC, Rashes, Eruptions, and Exanthems NEC
Investigations: Alkaline phosphatase increased, Bilirubin increased, Hepatic enzyme increased, Platelet count decreased, White blood cell count abnormal, severe hepatic injury
Musculoskeletal, Connective Tissue and Bone Disorders: Arthralgia, Myalgia, Muscle twitching, Rhabdomyolysis
Nervous System: Convulsion, Grand mal convulsion, Dyskinesia, serotonin syndrome in combination with serotonergic drugs
Psychiatric Disorders: Disorientation, Libido changes
Skin and Subcutaneous Tissue Disorders: Alopecia, Erythema
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of APTENSIO XR in pediatric patients under 6 years have not been established.
Safety and efficacy of APTENSIO XR were evaluated in a multicenter, placebo-controlled, double-blind, parallel group study in 119 children 4 to <6 years of age with ADHD followed by a 12-month open-label extension in 44 of these children. In these studies, patients experienced high rates of adverse reactions, most notably weight loss. Comparing weights prior to initiation of APTENSIO XR (in the safety and efficacy study) to weights after 12 months of treatment (in the open-label extension), 20 of 39 patients with data (50%) had lost enough weight to decrease 10 or more percentiles on a Centers for Disease Control growth chart for weight. In addition, systemic drug exposures in patients 4 to <6 years of age were higher than those observed in older children and adolescents at the same dose (2 to 3 fold higher Cmax and AUC). Therefore, the benefits of APTENSIO XR do not outweigh the risks in pediatric patients 4 to <6 years of age.
The safety and effectiveness of APTENSIO XR have been established in pediatric patients ages 6 to 17 years in two adequate and well-controlled clinical trials [see Clinical Studies (14)]. The long-term efficacy of methylphenidate in pediatric patients has not been established.
8.5 Geriatric Use
Clinical trials of APTENSIO XR did not include any patients aged 65 years and over. In general, dose selection for an elderly patient start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
9. Drug Abuse and Dependence
9.2 Abuse
CNS stimulants including APTENSIO XR, other methylphenidate-containing products, and amphetamines have a high potential for abuse. Abuse is characterized by impaired control over drug use despite harm, and craving.
Signs and symptoms of CNS stimulant abuse include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety, psychosis, hostility, aggression, suicidal or homicidal ideation have also been observed. Abusers of CNS stimulants may chew, snort, inject, or use other unapproved routes of administration which can result in overdose and death [see Overdosage (10)].
To reduce the abuse of CNS stimulants including APTENSIO XR, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse and on proper storage and disposal of CNS stimulants, monitor for signs of abuse while on therapy, and re-evaluate the need for APTENSIO XR use.
10. Overdosage
10.1 Signs and Symptoms
Signs and symptoms of acute methylphenidate overdose, resulting principally from overstimulation of the CNS and from excessive sympathomimetic effects, may include the following: nausea, vomiting, diarrhea, restlessness, anxiety, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, hypotension, tachypnea, mydriasis, dryness of mucous membranes, and rhabdomyolysis.
10.2 Management of Overdose
Consult with a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice on the management of overdosage with methylphenidate. Provide supportive care, including close medical supervision and monitoring. Treatment should consist of those general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdosages. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. Use supportive and symptomatic measures.
Gastric contents may be evacuated by gastric lavage as indicated. Before performing gastric lavage, control agitation and seizures if present and protect the airway. Other measures to detoxify the gut include administration of activated charcoal and a cathartic. Intensive care must be provided to maintain adequate circulation and respiratory exchange; external cooling procedures may be required for pyrexia.
11. Aptensio XR Description
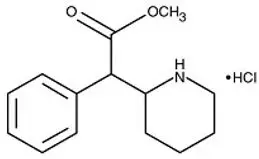
APTENSIO XR contains methylphenidate hydrochloride, a central nervous system (CNS) stimulant. APTENSIO XR capsules contain multi layered beads, which are composed of an immediate-release layer which contains approximately 40% of the methylphenidate dose, and a controlled release layer which contains approximately 60% of the methylphenidate dose. APTENSIO XR is available in seven capsule strengths. Each extended-release capsule for once-a-day oral administration contains 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 50 mg, or 60 mg of methylphenidate HCl USP, which is equivalent to 8.6 mg, 13.0 mg, 17.3 mg, 25.9 mg, 34.6 mg, 43.2 mg, or 51.9 mg of methylphenidate free base, respectively. Chemically, methylphenidate HCl is d,l (racemic) methyl α-phenyl-2-piperidineacetate hydrochloride. Its molecular formula is C14H19NO2∙HCl. Its structural formula is:
Methylphenidate hydrochloride USP is a white to off-white, odorless, fine crystalline powder. Its solutions are acid to litmus. It is freely soluble in water and in methanol, soluble in alcohol, and slightly soluble in chloroform and in acetone. Its molecular weight is 269.77.
Inactive Ingredients: ammonio methacrylate copolymer, type B; colloidal silicon dioxide (added if necessary); gelatin; hypromelloses; methacrylic acid copolymer, type C; polyethylene glycol; sugar spheres; talc; titanium oxide; and triethyl citrate.
Each strength capsule also contains colorant ingredients in the capsule shell as follows:
10 mg: FD&C Blue No. 1
15 mg: D&C Red No. 28, D&C Yellow No. 10, FD&C Red No. 40
20 mg: D&C Red No. 33, D&C Yellow No. 10
30 mg: FD&C Blue No. 1, FD&C Red No. 3
40 mg: D&C Red No. 28, FD&C Blue No. 1, FD&C Red No. 40
50 mg: D&C Yellow No. 10, FD&C Green No. 3
60 mg: Black Iron Oxide
12. Aptensio XR - Clinical Pharmacology
12.1 Mechanism of Action
Methylphenidate HCl is a central nervous system (CNS) stimulant. The mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Methylphenidate is a racemic mixture comprised of the d- and l-isomers. The d-isomer is more pharmacologically active than the l-isomer. Methylphenidate blocks the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
12.3 Pharmacokinetics
Absorption
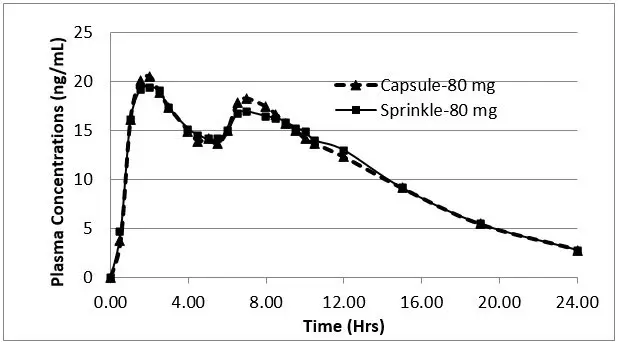
Following oral administration of APTENSIO XR in adults, plasma methylphenidate concentrations increase rapidly, reaching an initial maximum at about 2 hours, followed by gradual descending concentrations over the next 4 to 6 hours, after which a gradual increase begins, reaching a second peak at approximately 8 hours (Figure 1). The relative bioavailability of APTENSIO XR given once daily as compared to a methylphenidate immediate-release oral product given three times daily in adults is comparable. The relative bioavailability is 102%.
The pharmacokinetic profiles and parameters of methylphenidate are similar when APTENSIO XR is administered either as a whole capsule or sprinkled onto applesauce in subjects under fasting conditions (see Table 2 and Figure 1).
| Pharmacokinetic Parameters | Capsule | Sprinkle |
|---|---|---|
|
||
| Cmax†
(ng/mL) | 23.47 ± 11.4 | 21.78 ± 9.5 |
| AUC(0-t)†
(ng.hr/mL) | 262.7 ± 135 | 262.9 ± 128 |
| AUC(0-inf )†
(ng.hr/mL) | 258.1 ± 94.2 | 258.0 ± 84.4 |
| Tmax (hr) ‡ | 2.0 | 2.0 |
| Half-life (hr) | 5.09 | 5.43 |
| Relative bioavailability | 102% | 101% |
14. Clinical Studies
The efficacy of APTENSIO XR for the treatment of ADHD was established in a randomized, double-blind, single center, placebo-controlled, flexible-dose, cross-over trial in pediatric patients aged 6 to 12 years and a second randomized, double-blind, multicenter, placebo-controlled, fixed–dose trial in pediatric patients 6 to 17 years.
Pediatric Patients
A randomized, double-blind, placebo-controlled, flexible-dose, cross-over, analog classroom study (Study 1) was conducted in pediatric patients ages 6 to 12 years (N=26) who met DSM-IV-TR criteria for ADHD inattentive, hyperactive-impulsive or combined inattentive/hyperactive-impulsive subtypes.
Following a 2 to 4 week open-label dose optimization phase in which patients received flexible-dose APTENSIO XR 15 mg, 20 mg, 30 mg, or 40 mg administered once daily in the morning, patients were randomly assigned to APTENSIO XR (dose from open-label phase) or placebo. After 1-week of treatment, patients were evaluated over a period of 12 hours. Subsequently, patients were given the opposite treatment for 1-week and returned for the second evaluation. Patients could then enter an open-label extension phase for up to 21 months.
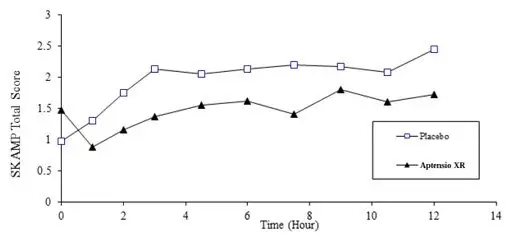
Efficacy assessments were conducted at 1, 2, 3, 4.5, 6, 7.5, 9, 10.5 and 12 hours post-dose using the Swanson, Kotkin, Agler, M. Flynn, and Pelham Total score (SKAMP). The primary efficacy endpoint was the average SKAMP Total Score, comparing APTENSIO XR to placebo. SKAMP is a validated 13-item teacher-rated scale that assesses manifestations of ADHD in a classroom setting.
The SKAMP Total Scores were statistically significantly better (lower) for APTENSIO XR than for placebo at the test day average and at all time points (1, 2, 3, 4.5, 6, 7.5, 9, 10.5 and 12 hours) post-dosing (see Figure 2).
Figure 2: Absolute SKAMP- Total Score after treatment with APTENSIO XR or Placebo (Study 1).
A randomized, double-blind, multicenter, placebo-controlled, parallel-group, fixed-dose study (Study 2) was conducted in pediatric patients age 6 to 17 years (N=230) who met DSM-IV-TR criteria for ADHD inattentive, hyperactive-impulsive or combined inattentive/hyperactive-impulsive subtypes.
The ADHD-RS-IV is an 18-item questionnaire with a score range of 0 to 54 points that measures the core symptoms of ADHD and includes both hyperactive/impulsive and inattentive subscales.
Patients were randomized to a daily morning dose of APTENSIO XR 10 mg, 15 mg, 20 mg, or 40 mg, or placebo for 1 week. An 11-week open label phase followed the double-blind phase. Patients could then enter another open-label phase for up to 21 months.
The primary efficacy endpoint was the mean decrease from baseline to the end of Week 1 in the ADHD-RS-IV Total Score. Each of the four APTENSIO XR doses (10 mg, 15 mg, 20 mg, and 40 mg/day) was compared to placebo at the end of week 1. For both the 20 mg/day and the 40 mg/day doses, APTENSIO XR was superior to placebo in reduction of the ADHD-RS-IV Total Score, but not for the 10 mg/day or the 15 mg/day doses.
A total of 221 patients completed the 1-week double-blind phase. Among those, 200 (90.5%) completed the 11-week open label phase and 173 (86.5%) patients continued into the 21-month open-label extension phase.
| Study Number | Treatment Group | Primary Efficacy Measure: ADHD-RS-IV Total Score | ||
|---|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Reduction from Baseline (SE) | Placebo-subtracted Difference* (95% CI) | ||
| Note: SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiple comparisons. |
||||
|
||||
| Study 2 (Pediatric) | APTENSIO XR 10 mg/day | 37.6 (8.32) | 9.1 (1.40) | 3.7 (-0.31, 7.66) |
| APTENSIO XR 15 mg/day | 38.0 (8.64) | 10.3 (1.59) | 4.9 (0.63, 9.07) | |
| APTENSIO XR 20 mg/day† | 36.2 (8.46) | 11.4 (1.49) | 6.0 (1.92, 10.02) | |
| APTENSIO XR 40 mg/day† | 35.6 (9.16) | 12.8 (1.49) | 7.4 (3.38, 11.45) | |
| Placebo | 33.4 (11.01) | 5.4 (1.48) | -- | |
16. How is Aptensio XR supplied
APTENSIO XR (methylphenidate hydrochloride extended-release) capsules are available as follows:
10 mg Capsules – light turquoise blue cap/white body, (imprinted with "APTENSIO XR" on cap and "10 mg" on the body)
| Bottles of 90 | NDC 42858-401-45 |
15 mg Capsules – orange cap/white body, (imprinted with "APTENSIO XR" on cap and "15 mg" on the body)
| Bottles of 90 | NDC 42858-402-45 |
20 mg Capsules – yellow cap/white body, (imprinted with "APTENSIO XR" on cap and "20 mg" on the body)
| Bottles of 90 | NDC 42858-403-45 |
30 mg Capsules – blue violet cap/white body, (imprinted with "APTENSIO XR" on cap and "30 mg" on the body)
| Bottles of 90 | NDC 42858-404-45 |
40 mg Capsules – pink cap/white body, (imprinted with "APTENSIO XR" on cap and "40 mg" on the body)
| Bottles of 90 | NDC 42858-405-45 |
50 mg Capsules – green cap/white body, (imprinted with "APTENSIO XR" on cap and "50 mg" on the body)
| Bottles of 90 | NDC 42858-406-45 |
60 mg Capsules – gray cap/white body, (imprinted with "APTENSIO XR" on cap and "60 mg" on the body)
| Bottles of 90 | NDC 42858-407-45 |
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 1/2023 |
| MEDICATION GUIDE APTENSIO XR® (App-ten-see-o) (methylphenidate hydrochloride extended-release) capsules, CII |
|
| What is the most important information I should know about APTENSIO XR? APTENSIO XR can cause serious side effects, including:
|
|
| What is APTENSIO XR?
APTENSIO XR is a central nervous system (CNS) stimulant prescription medicine used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in people 6 years of age and older. APTENSIO XR may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD.
|
|
Do not take APTENSIO XR if you or your child are:
|
|
Before taking APTENSIO XR tell your healthcare provider about all medical conditions, including if you or your child:
APTENSIO XR and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be changed during treatment with APTENSIO XR. Your healthcare provider will decide whether APTENSIO XR can be taken with other medicines. Especially tell your healthcare provider if you or your child take a medicine used to treat depression called monoamine oxidase inhibitor (MAOI). Know the medicines that you or your child take. Keep a list of the medicines with you to show your healthcare provider and pharmacist. Do not start any new medicine during treatment with APTENSIO XR without talking to your healthcare provider first. |
|
How should APTENSIO XR be taken?
|
|
| What should be avoided during treatment with APTENSIO XR?
Avoid drinking alcohol during treatment with APTENSIO XR. This may cause a faster release of the APTENSIO XR medicine. |
|
| What are possible side effects of APTENSIO XR? APTENSIO XR can cause serious side effects, including: See "What is the most important information I should know about APTENSIO XR?"
These are not all the possible side effects of APTENSIO XR. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Rhodes Pharmaceuticals at 1-888-827-0616. |
|
How should I store APTENSIO XR?
|
|
| General information about the safe and effective use of APTENSIO XR.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use APTENSIO XR for a condition for which it was not prescribed. Do not give APTENSIO XR to other people, even if they have the same symptoms. It may harm them and it is against the law. You can ask your doctor or pharmacist for information about APTENSIO XR that was written for healthcare professionals. |
|
| What are the ingredients in APTENSIO XR? Active Ingredient: methylphenidate hydrochloride Inactive Ingredients: ammonio methacrylate copolymer, type B; colloidal silicon dioxide (added if necessary); gelatin; hypromelloses; methacrylic acid copolymer, type C; polyethylene glycol; sugar spheres; talc; titanium oxide; and triethyl citrate. Manufactured by: Patheon Manufacturing Services LLC, Greenville, North Carolina 27834 For more information call Rhodes Pharmaceuticals (the distributor for APTENSIO XR) at 1-888-827-0616. |
|
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| APTENSIO XR
(methylphenidate hydrochloride) capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Rhodes Pharmaceuticals L.P. (831928986) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Manufacturing Services LLC | 079415560 | ANALYSIS(42858-401, 42858-402, 42858-403, 42858-404, 42858-405, 42858-406, 42858-407) , MANUFACTURE(42858-401, 42858-402, 42858-403, 42858-404, 42858-405, 42858-406, 42858-407) | |