Drug Detail:Arexvy (Respiratory syncytial virus vaccine, adjuvanted (rsv vaccine pref3, recombinant systemic))
Drug Class: Viral vaccines
Highlights of Prescribing Information
AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) suspension for intramuscular injection
Initial U.S. Approval: 2023
Indications and Usage for Arexvy
AREXVY is a vaccine indicated for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus in individuals 60 years of age and older. (1)
Arexvy Dosage and Administration
For intramuscular administration only.
Administer a single dose (0.5 mL) as an intramuscular injection. (2.1)
Dosage Forms and Strengths
Suspension for injection supplied as a single-dose vial of lyophilized antigen component to be reconstituted with the accompanying vial of adjuvant suspension component. A single dose after reconstitution is 0.5 mL. (3)
Contraindications
History of severe allergic reaction (e.g., anaphylaxis) to any component of the vaccine. (4)
Adverse Reactions/Side Effects
- •
- The most commonly reported solicited local adverse reaction (≥10%) was injection site pain (60.9%). (6.1)
- •
- The most commonly reported solicited systemic adverse reactions (≥10%) were fatigue (33.6%), myalgia (28.9%), headache (27.2%), and arthralgia (18.1%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2023
Full Prescribing Information
1. Indications and Usage for Arexvy
AREXVY is indicated for active immunization for the prevention of lower respiratory tract disease (LRTD) caused by respiratory syncytial virus in individuals 60 years of age and older.
2. Arexvy Dosage and Administration
2.2 Preparation
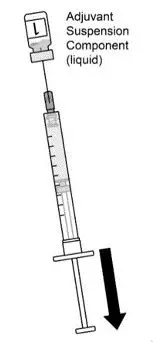
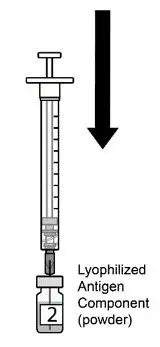
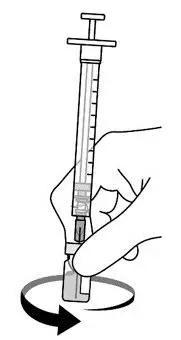
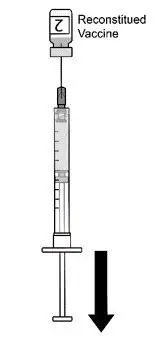
AREXVY is supplied in 2 vials that must be combined prior to administration. Prepare AREXVY by reconstituting the lyophilized antigen component (a sterile white powder) with the accompanying adjuvant suspension component (an opalescent, colorless to pale brownish sterile liquid). Use only the supplied adjuvant suspension component for reconstitution. The reconstituted vaccine should be an opalescent, colorless to pale brownish liquid. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered.
2.3 Administration
For intramuscular injection only.
After reconstitution, administer AREXVY immediately or store protected from light in the refrigerator between 2°C and 8°C (36°F to 46°F) or at room temperature [up to 25°C (77°F)] and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
3. Dosage Forms and Strengths
AREXVY is a suspension for injection supplied as a single-dose vial of lyophilized antigen component to be reconstituted with the accompanying vial of adjuvant suspension component. A single dose after reconstitution is 0.5 mL.
4. Contraindications
Do not administer AREXVY to anyone with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of AREXVY [see Description (11)].
5. Warnings and Precautions
5.1 Preventing and Managing Allergic Vaccine Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of AREXVY.
6. Adverse Reactions/Side Effects
In a clinical trial (NCT04886596), the most commonly reported (≥10%) adverse reactions were injection site pain (60.9%), fatigue (33.6%), myalgia (28.9%), headache (27.2%), and arthralgia (18.1%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of AREXVY was evaluated in 15,845 vaccine recipients.
Study 1 (NCT04886596) is a placebo-controlled, Phase 3 clinical study conducted in Europe, North America, Asia, and the Southern Hemisphere (South Africa, Australia, and New Zealand), involving 24,966 participants, 60 years of age and older, who received AREXVY (n = 12,467) or saline placebo (n = 12,499). Study 2 (NCT04732871) is a non-placebo-controlled, open-label, Phase 3 clinical study conducted in Europe, North America, and Asia, involving 1,653 participants, 60 years of age and older, who received AREXVY. Study 3 (NCT04841577) is a non-placebo-controlled, open-label, Phase 3 clinical study conducted in New Zealand, Panama, and South Africa, involving participants 60 years of age and older who received 1 dose of AREXVY and FLUARIX QUADRIVALENT concomitantly (n = 442) or sequentially (n = 443).
At the time of vaccination in Study 1, the median age of the population was 69.0 years; 13,943 (55.8%) participants were 60 to 69 years of age, 8,978 (36.0%) participants were 70 to 79 years of age, and 2,045 (8.2%) participants were 80 years of age and older. The majority of participants were White (79.4%), followed by Black (8.7%), Asian (7.6%), and other racial/ethnic groups (4.3%); 5.5% were of Hispanic or Latino ethnicity; 51.7% were female. In Study 2, the median age of the population at the time of vaccination was 69.0 years; 820 (49.6%) participants were 60 to 69 years of age, 621 (37.6%) participants were 70 to 79 years of age, and 212 (12.8%) participants were 80 years of age and older. In Study 2, the majority of participants were White (67.8%), followed by Asian (30.0%), Black (2.0%), and other racial/ethnic groups (0.2%); 1.9% were of Hispanic or Latino ethnicity; 54.6% were female. In Study 3, the median age of the population at the time of the vaccination was 67.0 years; 519 (58.6%) participants were 60 to 69 years of age, 288 (32.5%) participants were 70 to 79 years of age, and 78 (8.8%) participants were 80 years of age and older, respectively. In Study 3, the majority of the participants were of mixed race (50.3%), followed by White (30.7%), and Black (16.0%); 34.7% were of Hispanic or Latino ethnicity; 51.5% were female.
Safety Data from Study 1
Solicited Adverse Reactions: In Study 1, a subset of study participants (solicited safety set) was monitored for solicited adverse reactions using standardized paper diary cards during the 4 days (i.e., day of vaccination and the next 3 days) following a dose of AREXVY or placebo; 879 participants received AREXVY and 874 participants received placebo. The other study participants did not prospectively record solicited reactions on a diary card but may have reported them as unsolicited adverse reactions.
The reported frequencies of specific solicited local (administration site) and systemic adverse reactions (per participant) are presented in Table 1.
| N = Exposed set for solicited safety set included all participants with at least 1 documented dose. a Placebo was a saline solution. b Any grade pain: Defined as any pain neither interfering with nor preventing normal everyday activities (Grade 1), painful when limb is moved and interferes with everyday activities (Grade 2), or significant pain at rest and prevents normal everyday activities (Grade 3). c Any grade fatigue, myalgia, headache, arthralgia: Defined as event easily tolerated (Grade 1), interfering with normal activity (Grade 2), or preventing normal activity (Grade 3). d Temperature taken by any route (oral, axillary, or tympanic). |
||
|
AREXVY % |
Placeboa % |
|
|
Local Adverse Reactions |
N = 879 |
N = 874 |
|
Pain, Anyb |
60.9 |
9.3 |
|
Pain, Grade 3b |
1 |
0 |
|
Erythema, >20 mm |
7.5 |
0.8 |
|
Erythema, >100 mm |
0.2 |
0 |
|
Swelling, >20 mm |
5.5 |
0.6 |
|
Swelling, >100 mm |
0.2 |
0 |
|
Systemic Adverse Reactions |
N = 879 |
N = 878 |
|
Fatigue, Anyc |
33.6 |
16.1 |
|
Fatigue, Grade 3c |
1.7 |
0.5 |
|
Myalgia, Anyc |
28.9 |
8.2 |
|
Myalgia, Grade 3c |
1.4 |
0.3 |
|
Headache, Anyc |
27.2 |
12.6 |
|
Headache, Grade 3c |
1.3 |
0 |
|
Arthralgia, Anyc |
18.1 |
6.4 |
|
Arthralgia, Grade 3c |
1.3 |
0.6 |
|
Fever, ≥38.0°C/100.4°Fd |
2.0 |
0.3 |
|
Fever, >39.0°C/102.2°Fd |
0.1 |
0.1 |
In the solicited safety set, the local administration site adverse reactions reported with AREXVY had a median duration of 2 days, and the systemic adverse reactions reported with AREXVY had a median duration ranging between 1 and 2 days.
Unsolicited Adverse Events: In all participants from Study 1, unsolicited adverse events were monitored using paper diary cards during the 30-day period following vaccination (day of vaccination and the next 29 days).
Among participants in the solicited safety set, (AREXVY, n = 879 or placebo, n = 878), unsolicited adverse events occurring within 30 days after vaccination were reported in 14.9% and 14.6% of participants who received AREXVY and placebo, respectively.
In the exposed set, 24,966 participants 60 years of age and older, received at least 1 dose of AREXVY (n = 12,467) or placebo (n = 12,499). Unsolicited adverse events occurring within 30 days of vaccination were reported in 33.0% and 17.8% of participants, respectively. The higher frequency of reported unsolicited adverse events among participants who received AREXVY, compared to participants who received placebo, was primarily attributed to events that are consistent with adverse reactions solicited among participants in the reactogenicity subset. Within 30 days after vaccination, atrial fibrillation was reported in 10 participants who received AREXVY and 4 participants who received placebo (of which 7 events in AREXVY arm and 1 event in placebo arm were serious); the onset of symptoms ranged from 1 to 30 days post vaccination. The currently available information on the atrial fibrillation is insufficient to determine a causal relationship to the vaccine. There were no other notable patterns or numerical imbalances between groups for specific categories of unsolicited adverse events.
Serious Adverse Events: In Study 1, participants were monitored for all serious adverse events (SAEs) that occurred during the 6‑month period following administration of AREXVY (n = 12,467) or placebo (n = 12,499).
SAEs with onset within 6 months following vaccination were reported at similar rates in participants who received AREXVY (4.2%) or placebo (4.0%). Serious events of atrial fibrillation were reported in 13 participants who received AREXVY and 15 participants who received placebo within 6 months after vaccination.
Deaths: From vaccination through the first analysis of the ongoing Study 1, adverse events leading to death were reported for 49 participants (0.4%) who received AREXVY (n = 12,467) and 58 participants (0.5%) who received placebo (n = 12,499). Based on available information, there is no evidence of causal relationship to AREXVY. Causes of death among participants were consistent with those generally reported in adult and elderly populations.
Potential Immune-Mediated Diseases: In Study 1, participants were monitored for all potential immune-mediated diseases (pIMDs) that occurred during the 6-month period following administration of AREXVY (n = 12,467) or placebo (n = 12,499).
New onset pIMDs or exacerbation of existing pIMDs within 6 months following vaccination were reported for 0.3% of participants who received AREXVY and 0.3% of participants who received placebo. There were no notable imbalances between study groups in individual pIMDs reported.
Serious Adverse Events Reported From Other Studies
Study 2: Guillain-Barré syndrome beginning 9 days after AREXVY vaccination was reported in a participant enrolled in a study site in Japan.
Study 3: Acute disseminated encephalomyelitis was reported in 2 participants enrolled in a study site in South Africa; the onset of the symptoms was 7 and 22 days post vaccination, respectively. One event was fatal and the other non-fatal. These participants received AREXVY concomitantly with FLUARIX QUADRIVALENT.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
AREXVY is not approved for use in persons <60 years of age.
In a clinical study that enrolled pregnant individuals who received an investigational unadjuvanted RSV vaccine that contained the same RSVPreF3 antigen as AREXVY, an increase in preterm births was observed compared to pregnant individuals who received placebo (sucrose reconstituted with saline).
Data
In a randomized controlled clinical trial that enrolled pregnant individuals in a 2:1 ratio, 3,557 received an investigational unadjuvanted RSV vaccine that contained the same RSVPreF3 antigen as AREXVY and 1,771 received placebo (sucrose reconstituted with saline) at 24 to 34 weeks gestation. In the vaccine and placebo groups, 6.81% and 4.95% of preterm births were reported, respectively.
8.2 Lactation
Risk Summary
It is not known whether AREXVY is excreted in human milk. AREXVY is not approved for use in persons <60 years of age. No human or animal data are available to assess the effects of AREXVY on the breastfed infant or on milk production/excretion.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AREXVY and any potential adverse effects on the breastfed child from AREXVY or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Evidence from an animal model strongly suggests that AREXVY would be unsafe in individuals younger than 2 years of age because of an increased risk of enhanced respiratory disease. Safety and effectiveness in individuals 2 years through 17 years of age have not been established.
8.5 Geriatric Use
AREXVY is approved for use in individuals 60 years of age and older. Of the total number of participants (N = 24,966) who received AREXVY or placebo in Study 1 (NCT04886596), 13,943 (55.8%) were 60 to 69 years of age, 8,978 (36.0%) were 70 to 79 years of age, and 2,045 (8.2%) were 80 years of age and older. [see Adverse Reactions (6.1), Clinical Studies (14.1)].
11. Arexvy Description
AREXVY (Respiratory Syncytial Virus Vaccine, Adjuvanted) is a sterile suspension for intramuscular injection. The vaccine is supplied as a vial of lyophilized recombinant respiratory syncytial virus glycoprotein F stabilized in pre-fusion conformation (RSVPreF3) as the antigen component, which must be reconstituted at the time of use with the accompanying vial of AS01E adjuvant as the adjuvant suspension component.
The RSVPreF3 antigen is expressed by culturing genetically engineered Chinese Hamster Ovary cells in media containing no antibiotics or animal-derived proteins. The RSVPreF3 protein is purified by several chromatographic and filtration steps, formulated with excipients, filled into vials, and lyophilized.
The AS01E adjuvant is composed of 3‑O‑desacyl‑4’‑monophosphoryl lipid A (MPL) from Salmonella minnesota and QS-21, a saponin purified from plant extract Quillaja saponaria Molina, combined in a liposomal formulation. The liposomes are composed of dioleoyl phosphatidylcholine (DOPC) and cholesterol in a phosphate-buffered saline solution containing disodium phosphate anhydrous, potassium dihydrogen phosphate, sodium chloride, and water for injection.
After reconstitution, each 0.5-mL dose is formulated to contain 120 mcg of the recombinant RSVPreF3 antigen, 25 mcg of MPL, and 25 mcg of QS-21. Each dose also contains 14.7 mg of Trehalose, 4.4 mg of sodium chloride, 0.83 mg of potassium dihydrogen phosphate, 0.26 mg of dipotassium phosphate, 0.18 mg of polysorbate 80, 0.15 mg of disodium phosphate anhydrous, 0.5 mg of DOPC, and 0.125 mg of cholesterol.
AREXVY contains no preservative. Each dose may also contain residual amounts of host cell proteins (≤2.0%) and DNA (≤0.80 ng/mg) from the manufacturing process.
The vial stoppers are not made with natural rubber latex.
14. Clinical Studies
14.1 Efficacy in Adults 60 Years of Age and Older
Efficacy of AREXVY against RSV-associated LRTD in adults 60 years of age and older was evaluated in Study 1 (NCT04886596), an ongoing, Phase 3, randomized, placebo‑controlled, observer-blind clinical study conducted in 17 countries from Northern and Southern Hemispheres. Participants are planned to be followed for up to 36 months.
The study excluded participants who were immunocompromised. Participants with pre-existing, chronic, stable disease such as diabetes, hypertension, or cardiac disease were allowed to participate in the study if considered by the investigator as medically stable at the time of vaccination.
The primary population for efficacy analysis (referred to as the modified exposed set, included adults 60 years of age and older receiving 1 dose of AREXVY or placebo and who did not report an RSV-confirmed acute respiratory illness [ARI] prior to Day 15 after vaccination) included 24,960 participants randomized equally to receive 1 dose of AREXVY (n = 12,466) or placebo (n = 12,494). At the time of the primary efficacy analysis, participants had been followed for the development of RSV-associated LRTD for up to 10 months (median of 6.7 months).
At the time of first efficacy analysis of the ongoing Study 1, 51.7% were female; 79.4% were White, 8.7% were Black, 7.6% were Asian, and 4.3% were of other racial/ethnic groups including American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander; 5.5% were of Hispanic or Latino ethnicity. The median age of participants was 69.0 years.
At baseline, 39.3% of participants had at least one comorbidity of interest; 19.7% of participants had an underlying cardiorespiratory condition (chronic obstructive pulmonary disease, asthma, any chronic respiratory/pulmonary disease, or chronic heart failure) and 25.8% of participants had endocrine and metabolic conditions (diabetes, advanced liver or renal disease).
Efficacy against Respiratory Syncytial Virus-associated Lower Respiratory Tract Disease
The primary objective was to demonstrate the efficacy of AREXVY in the prevention of a first episode of confirmed RSV-A and/or B‑associated LRTD during the first season.
Confirmed RSV cases were determined by quantitative Reverse Transcription Polymerase Chain Reaction (qRT‑PCR) on a nasopharyngeal swab during all ARI episodes. Acute respiratory illness (ARI) was defined by the presence of at least 2 respiratory symptoms/signs for at least 24 hours (nasal congestion, sore throat, lower respiratory symptoms/signs, as described below), or at least 1 respiratory symptom/sign plus 1 systemic symptom/sign (fever or feverishness, fatigue, body aches, headache, decreased appetite) for at least 24 hours. LRTD was defined based on the following criteria: the participant must have experienced at least 2 lower respiratory symptoms/signs, including at least 1 lower respiratory sign for at least 24 hours, or experienced at least 3 lower respiratory symptoms for at least 24 hours. Lower respiratory symptoms included: new or increased sputum, new or increased cough, new or increased dyspnea (shortness of breath). Lower respiratory signs included: new or increased wheezing, crackles/rhonchi, respiratory rate ≥20 respirations/min, low or decreased oxygen saturation (O2 saturation <95% or ≤90% if baseline is <95%), need for oxygen supplementation.
Compared with placebo, AREXVY significantly reduced the risk of developing RSV‑associated LRTD by 82.6% (96.95% CI [57.9, 94.1]) in participants 60 years of age and older, which met the pre-specified success criterion for the primary study objective (Table 2). The median duration of efficacy follow-up was 6.7 months.
Vaccine efficacy analyses by age subgroup and for participants with at least one comorbidity of interest are presented in Table 2.
The vaccine efficacy against RSV A-associated LRTD cases and RSV B-associated LRTD cases was 84.6% (95% CI [32.1, 98.3]) and 80.9% (95% CI [49.4, 94.3]), respectively.
| Two-sided exact CI for vaccine efficacy is derived based on Poisson model adjusted by age categories and regions. N = Number of participants included in each group. n = Number of participants having first occurrence of RSV-confirmed LRTD occurring from Day 15 post-vaccination. a Study 1: NCT04886596. b CI = Confidence Interval (96.95% for the overall ≥60 years and 95% for all subgroup analyses). |
|||||||
|
Subgroup |
AREXVY |
Placebo |
% Efficacy (CI)b |
||||
|
N |
n |
Incidence Rate per 1,000 Person-Years |
N |
n |
Incidence Rate per 1,000 Person-Years |
||
|
Overall
|
12,466 |
7 |
1.0 |
12,494 |
40 |
5.8 |
82.6 (57.9, 94.1) |
|
6,963 |
4 |
1.0 |
6,979 |
21 |
5.5 |
81.0 (43.6, 95.3) |
|
4,487 |
1 |
0.4 |
4,487 |
16 |
6.5 |
93.8 (60.2, 99.9) |
|
Participants with at least 1 comorbidity of interest |
4,937 |
1 |
0.4 |
4,861 |
18 |
6.6 |
94.6 (65.9, 99.9) |
Compared with placebo, AREXVY significantly reduced the risk of developing RSV‑associated LRTD by 84.4% (95% CI [46.9, 97.0]) in participants 70 years of age and older. The vaccine efficacy in the subgroup of participants 80 years of age and older (1,016 participants who received AREXVY versus 1,028 participants who received placebo) cannot be concluded due to the low number of total cases accrued (2 cases among participants who received AREXVY and 3 cases among participants who received placebo).
Efficacy Against Severe Respiratory Syncytial Virus-associated Lower Respiratory Tract Disease
In Study 1, a severe RSV-associated LRTD was defined as an RT-PCR confirmed RSV‑associated LRTD with at least 2 lower respiratory signs, or as an RT-PCR confirmed RSV‑associated LRTD episode preventing normal, everyday activities. One case of severe RSV‑associated LRTD in the group that received AREXVY and 17 cases in the group that received placebo were reported, amongst which 2 cases required supportive therapy. Compared with placebo, AREXVY significantly reduced the risk of developing severe RSV‑associated LRTD by 94.1% (95% CI [62.4, 99.9]) in participants 60 years of age and older.
14.2 Concomitant Administration
In Study 3 (NCT04841577), an open-label, Phase 3, clinical study conducted in New Zealand, Panama, and South Africa, participants 60 years of age and older received 1 dose of AREXVY and FLUARIX QUADRIVALENT at Month 0 (n = 442) or 1 dose of FLUARIX QUADRIVALENT at Month 0 followed by a dose of AREXVY at Month 1 (n = 443).
There was no evidence for interference in the immune response to any of the antigens contained in both concomitantly administered vaccines. The criteria for non-inferiority of the immune responses in the control versus “co-administration” group were met as the 2-sided 95% confidence interval upper limits on the group geometric mean titer ratios were below 1.5 for the RSV-A neutralizing antibodies and haemagglutinin inhibition antibodies against the strains Flu A/Hong Kong/H3N2, Flu A/Victoria/H1N1, Flu B/Phuket/Yamagata, and Flu B/Washington/Victoria.
Data are not available for concomitant administration with other vaccines.
16. How is Arexvy supplied
AREXVY is supplied as 2 components: A single-dose vial of lyophilized antigen component (powder) and a single-dose vial of adjuvant suspension component (liquid) (packaged without syringes or needles).
|
Presentation |
Carton NDC Number |
Components |
|
|
Adjuvant Suspension Component (liquid) |
Lyophilized Antigen Component (powder) |
||
|
Outer carton of 10 doses |
58160-848-11 |
10 vials NDC 58160-744-03 |
10 vials NDC 58160-723-03 |
16.1 Storage before Reconstitution
Adjuvant suspension component vials: Store refrigerated between 2°C and 8°C (36°F and 46°F). Store in the original package in order to protect vials from light. Do not freeze. Discard if the adjuvant suspension component has been frozen.
Lyophilized antigen component vials: Store refrigerated between 2°C and 8°C (36°F and 46°F). Store in the original package in order to protect vials from light. Do not freeze. Discard if the antigen component has been frozen.
16.2 Storage after Reconstitution
- •
- Administer immediately or store in the refrigerator between 2°C and 8°C (36°F to 46°F) or at room temperature [up to 25°C (77°F)] for up to 4 hours prior to use.
- •
- Protect vials from light.
- •
- Discard reconstituted vaccine if not used within 4 hours.
- •
- Do not freeze. Discard if the vaccine has been frozen.
17. Patient Counseling Information
- •
- Inform vaccine recipients of the potential benefits and risks of vaccination with AREXVY.
- •
- Inform vaccine recipients about the potential for adverse reactions that have been observed following administration of AREXVY.
- •
- Provide the Vaccine Information Statements, which are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured by GlaxoSmithKline Biologicals
Rixensart, Belgium, U.S. License 1617, and
Distributed by GlaxoSmithKline
Durham, NC 27701
©2023 GSK group of companies or its licensor.
ARV:1PI
PRINCIPAL DISPLAY PANEL
NDC 58160-848-11
AREXVY
Respiratory Syncytial Virus Vaccine, Adjuvanted
Rx only
For 60 Years of Age and Older
Contents (10 doses fo AREXVY):
10 Vials containing Adjuvant Suspension Component
10 Vials containing Lyophilized Antigen Component
After reconstitution, a single dose of AREXVY is 0.5 mL
GSK
AREXVY
Adjuvant and Antigen Made in Belgium
©2023 GSK group of companies or its licensor.
Rev. 04/23
513500
PRINCIPAL DISPLAY PANEL (Antigen)
NDC 58160-723-03
Lyophilized Antigen Component
Vial 2 of 2
NOT TO BE USED ALONE
Reconstitute with Adjuvant Suspension Component to form Respiratory Syncytial Virus Vaccine, Adjuvanted (AREXVY)
0.5-mL Single Dose After Reconstitution
Mfd. By GlaxoSmithKline Biologicals
Rev 04/23
513499
| AREXVY
respiratory syncytial visus vaccine recombinant, adjuvanted kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GlaxoSmithKline Biologicals SA (372748392) |
Medical Disclaimer